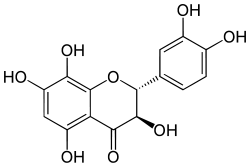
Dihydrogossypetin
Dihydrogossypetin is a flavanonol, a type of flavonoid.[1]
 | |
| Names | |
|---|---|
| IUPAC name
(2R,3R)-2-(3,4-dihydroxyphenyl)-3,5,7,8-tetrahydroxy-2,3-dihydrochromen-4-one | |
| Other names
2,3-Dihydrogossypetin 3′,4′,5,7,8-pentahydroxyflavanonol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C15H12O8 | |
| Molar mass | 320.25 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biosynthesis
The enzyme Taxifolin 8-monooxygenase hydroxylates taxifolin using NADH, NADPH, H+, and O2 to produce 2,3-dihydrogossypetin, NAD+, NADP+, and H2O.
gollark: It will just implicitly cast things all over the place.
gollark: I agree. Making Macron more like Haskell has to be a good thing.
gollark: Perl is retroactively Macron?!
gollark: It's too complicated, says basically nothing about Macron, and is just ugly and beeoidal (class φ-7).
gollark: The Committee is bikeshedding horribly wrt. logo.
References
- Jeffrey, A. M.; Jerina, D. M.; Self, R.; Evans, W. C. (1972). "The bacterial degradation of flavonoids. Oxidative fission of the A-ring of dihydrogossypetin by a Pseudomonas sp". The Biochemical Journal. 130 (2): 383–90. doi:10.1042/bj1300383. PMC 1174417. PMID 4198081.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.