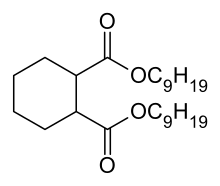
1,2-Cyclohexane dicarboxylic acid diisononyl ester
1,2-Cyclohexane dicarboxylic acid diisononyl ester is a plasticizer for the manufacture of flexible plastic articles in sensitive application areas such as toys, medical devices and food packaging. From a chemical point of view it belongs to the group of aliphatic esters.
 | |
| Names | |
|---|---|
| IUPAC name
Diisononyl cyclohexane-1,2-dicarboxylate | |
| Other names
Cyclohexane-1,2-dicarboxylic acid diisononyl ester; Hexamoll DINCH (tradename). | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.121.507 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C26H48O4 | |
| Molar mass | 424.666 g·mol−1 |
| Appearance | colorless liquid[1] |
| Odor | almost odorless[1] |
| Density | 0.944–0.954 g·cm−3[1] |
| Melting point | Pour point: −54 °C (−65 °F; 219 K)[1] |
| Hazards | |
| Safety data sheet | BASF Safety Data Sheet |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
In 2002 BASF started selling 1,2-cyclohexane dicarboxylic acid diisononyl ester under the tradename of Hexamoll® DINCH as an alternative for phthalate plasticizers.[2]
Production
The two commercial routes to manufacture 1,2-cyclohexane dicarboxylic acid diisononyl ester are the catalytic hydrogenation of diisononyl phthalate[3][4] and the Diels-Alder reaction of a maleic acid ester with 1,3-butadiene followed by hydrogenation. In the case of the catalytic hydrogenation the aromatic, planar part of the diisononyl phthalate is transformed to a cyclohexane ring by a formal addition of 6 hydrogen atoms while the alkyl and ester groups are not affected by the hydrogenation. Based on the catalysator used, the resulting product consists of 90% of the cis and 10% of the trans isomer of the cyclohexane-1,2-dicarboxlic acid diisononylester.
Regulatory approval
Food contact
In the European Union the European Food Safety Authority has approved 1,2-cyclohexane dicarboxylic acid diisononyl ester for a wide variety of food contact applications in October 2006.[5] In 2007 1,2-cyclohexane dicarboxylic acid diisononyl ester has been added to Annex III of the "Directive 2002/72/EC relating to plastic materials and articles intended to come into contact with food".[6] The EU Directive 2002/72 has meanwhile been superseded by Regulation (EU) No 10/2011.[7] Hexamoll® DINCH is listed in Annex I of Regulation (EU) No 10/2011 by as Food Conctact Material (FCM) 775 by its chemical name, i.e. as 1,2-cyclohexanedicarboxylic acid, diisononylester.
Toys
A US federal law was passed in 2008 banning the use of some phthalates in children's toys.[8] When the law took effect in February 2009, Mattel and Learning Curve confirmed to NPR they were substituting phthalates with Hexamoll® DINCH and citrate-based plasticizers.[9]
In the European Union 1,2-cyclohexane dicarboxylic acid diisononyl ester was not listed in directive 2005/84/EC. These restrictions for certain phthalates are now transferred into Annex XVII, 51 and 52 of Regulation (EC) No 1907/2006 which ban the use of certain phthalates in toys and childcare articles and thus DINCH can be used safely in toys and childcare articles.[10]
Possible Health Effects
A research group from Harvard and CDC found suggestive negative associations between urinary MHiNCH, the monoester of DINCH, a minor urinary metabolite. . Their research at a fertility clinic showed that women who had been exposed to DINCH had lower, but statistically not significant estradiol hormone levels and fewer, again not statistically significant number of oocytes in their ovaries. However, urinary MHiNCH concentrations were unrelated to mature oocyte yield and endometrial wall thickness.[11] The results are inconclusive and therefore, the authors suggest that more epidemiological studies would be needed for clarification.
The Swedish IVL Environmental Research Institute and researches from the Department of Environmental Science and Analytical Chemistry of Stockholm University recommend that "children's exposure to DINCH should be investigated in more detail and exposure to the general population should be closely monitored"[12] as DINCH is used as an alternative plasticizer in e.g. children's toys.
The Chronic Hazard Advisory Panel of the U.S. Consumer Product Safety Commission "strongly encourages the appropriate U.S. agencies to obtain the necessary toxicological and exposure data to assess any potential risk from DINX" because of "lack of publicly available information".[13][14]
A French group from the University of Clermont-Ferrand noted that clinical data and data regarding the migration from Medical Devices would be rare. The Project was funded by the French Agency for the Safety of Health Products (ANSM). It remains unclear why this Research Group was not able to identify the published human biomonitoring data which give a perfect overview on population exposure of alternative plasticizers (e.g. DINCH/DINX) and their metabolites.[15]
A report by the Danish Ministry of the Environment states that the available data for DINCH do not indicate a need for further investigations. Further, the conclusion is that 3 of the evaluated plasticizers, namely the substances COMGHA, DEHT and DINCH may be seen as the most promising alternatives, as these substances have an extended data set (complying to Annex X data requirements, i.e. a two-generation reproduction study) without indicating specific concern for reproductive toxicity or endocrine activity.[16] Toxicogenomic screening showed that 648 genes were significantly changed after 48 hours exposure to DINCH suggesting that "DINCH is biologically active".[17]
References
- BASF Technical Leaflet Hexamoll DINCH
- Flexible vinyls get new non-phthalate plasticizer. (Keeping Up With Additives). (September 1, 2002). Plastics Technology. Allbusiness.com. Accessed 2009-02-12.
- Innovative plasticizer alternative to phthalates for non-PVC applications
- Patent WO 99/32427, "Verfahren zur Hydrierung von Benzolpolycarbonsäuren oder Derivaten davon unter Verwendung eines Makroporen aufweisenden Katalysators", July 1, 1999, BASF AG
- EFSA: Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request related to a 12th list of substances for food contact materials, EFSA-Journal, 2006, 395–401, 1–21.
- Official Journal of the European Union: Corrigendum to Commission Directive 2007/19/EC of 30 March 2007 amending Directive 2002/72/EC relating to plastic materials and articles intended to come into contact with food and Council Directive 85/572/EEC laying down the list of simulants to be used for testing migration of constituents of plastic materials and articles intended to come into contact with foodstuffs, April 2, 2007.
- COMMISSION REGULATION (EU) No 10/2011 of 14 January 2011 on plastic materials and articles intended to come into contact with food Accessed 2013-08-05
- Brett Clanton (August 11, 2008). Federal ban on a chemical used in toys will affect Texas. Houston Chronicle. Accessed 2009-02-12.
- Sarah Varney (February 12, 2009). New Safety Law Doesn't Mean All's Well In Toyland. NPR. Accessed 2009-02-12.
- Official Journal of the European Union: Directive 2005/84/EC of the European Parliament and of the Council of 14 December 2005 amending for the 22nd time Council Directive 76/769/EEC on the approximation of the laws, regulations and administrative provisions of the Member States relating to restrictions on the marketing and use of certain dangerous substances and preparations (phthalates in toys and childcare articles)
- Mínguez-Alarcón, Lidia; Souter, Irene; Chiu, Yu-Han; Williams, Paige L.; Ford, Jennifer B.; Ye, Xiaoyun; Calafat, Antonia M.; Hauser, Russ (2016-11-01). "Urinary concentrations of cyclohexane-1,2-dicarboxylic acid monohydroxy isononyl ester, a metabolite of the non-phthalate plasticizer di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH), and markers of ovarian response among women attending a fertility center". Environmental Research. 151: 595–600. doi:10.1016/j.envres.2016.08.012. PMC 5071161. PMID 27591839.
- Bui, Thuy T.; Giovanoulis, Georgios; Cousins, Anna Palm; Magnér, Jörgen; Cousins, Ian T.; de Wit, Cynthia A. (2016-01-15). "Human exposure, hazard and risk of alternative plasticizers to phthalate esters". Science of the Total Environment. 541: 451–467. doi:10.1016/j.scitotenv.2015.09.036. PMID 26410720.
- Lioy, Paul J; Hauser, Russ; Gennings, Chris; Koch, Holger M; Mirkes, Philip E; Schwetz, Bernard A; Kortenkamp, Andreas (2015). "Assessment of phthalates/phthalate alternatives in children's toys and childcare articles: Review of the report including conclusions and recommendation of the Chronic Hazard Advisory Panel of the Consumer Product Safety Commission". Journal of Exposure Science and Environmental Epidemiology. 25 (4): 343–353. doi:10.1038/jes.2015.33.
- "Report to the U.S. Consumer Product Safety Commission by the CHRONIC HAZARD ADVISORY PANEL ON PHTHALATES AND PHTHALATE ALTERNATIVES" (PDF). United States Consumer Product Safety Commission. 2014.
- Bernard, L.; Décaudin, B.; Lecoeur, M.; Richard, D.; Bourdeaux, D.; Cueff, R.; Sautou, V. (2014-11-01). "Analytical methods for the determination of DEHP plasticizer alternatives present in medical devices: A review". Talanta. 129: 39–54. doi:10.1016/j.talanta.2014.04.069.
- B. S. Nielsen; D. Nørgaard Andersen; E. Giovalle; M. Bjergstrøm; P.B. Larsen (2014). "Alternatives to classified phthalates in medical devices, Danish Environmental Protection Agency, Environmental Project No. 1557, 2014, Copenhagen" (PDF).
- Nardelli, Thomas C.; Erythropel, Hanno C.; Robaire, Bernard (2015-10-07). "Toxicogenomic Screening of Replacements for Di(2-Ethylhexyl) Phthalate (DEHP) Using the Immortalized TM4 Sertoli Cell Line". PLOS ONE. 10 (10): e0138421. doi:10.1371/journal.pone.0138421. ISSN 1932-6203. PMC 4596883. PMID 26445464.