Precipitation (chemistry)
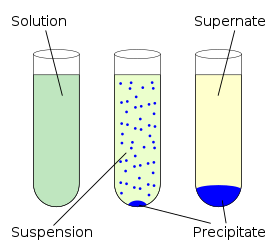
Precipitation defined as the creation of a solid from a solution. When the reaction occurs in a liquid solution, the solid formed is called the 'precipitate'. The chemical that causes the solid to form is called the 'precipitant'. Without sufficient force of gravity (settling) to bring the solid particles together, the precipitate remains in suspension. After sedimentation, especially when using a centrifuge to press it into a compact mass, the precipitate may be referred to as a 'pellet'. Precipitation can be used as a medium. The precipitate-free liquid remaining above the solid is called the 'supernate' or 'supernatant'. Powders derived from precipitation have also historically been known as 'flowers'. When the solid appears in the form of cellulose fibers which have been through chemical processing, the process is often referred to as regeneration.
Sometimes the formation of a precipitate indicates the occurrence of a chemical reaction. If silver nitrate solution is poured into a solution of sodium chloride, a chemical reaction occurs forming a white precipitate of silver chloride. When potassium iodide solution reacts with lead(II) nitrate solution, a yellow precipitate of lead(II) iodide is formed.
The precipitation may occur if concentration of a compound exceeds its solubility (such as when mixing solvents or changing their temperature). Precipitation may also occur rapidly from a supersaturated solution.
In solids, precipitation occurs if the concentration of one solid is above the solubility limit in the host solid, due to e.g. rapid quenching or ion implantation, and the temperature is high enough that diffusion can lead to segregation into precipitates. Precipitation in solids is routinely used to synthesize nanoclusters.[1]
An important stage of the precipitation process is the onset of nucleation. The creation of a hypothetical solid particle includes the formation of an interface, which requires some energy based on the relative surface energy of the solid and the solution. If this energy is not available, and no suitable nucleation surface is available, supersaturation occurs.
Applications


Precipitation reactions can be used for making pigments, removing salts from water in water treatment, and in classical qualitative inorganic analysis.
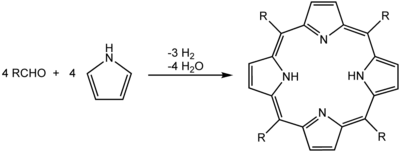
Precipitation is also useful to isolate the products of a reaction during workup. Ideally, the product of the reaction is insoluble in the reaction solvent. Thus, it precipitates as it is formed, preferably forming pure crystals. An example of this would be the synthesis of porphyrins in refluxing propionic acid. By cooling the reaction mixture to room temperature, crystals of the porphyrin precipitate, and are collected by filtration:[2]
Precipitation may also occur when an antisolvent (a solvent in which the product is insoluble) is added, drastically reducing the solubility of the desired product. Thereafter, the precipitate may easily be separated by filtration, decanting, or centrifugation. An example would be the synthesis of chromic tetraphenylporphyrin chloride: water is added to the DMF reaction solution, and the product precipitates.[3] Precipitation is also useful in purifying products: crude bmim-Cl is taken up in acetonitrile, and dropped into ethyl acetate, where it precipitates.[4] Another important application of an antisolvent is in ethanol precipitation of DNA.
In metallurgy, precipitation from a solid solution is also a useful way to strengthen alloys; this process is known as solid solution strengthening.
Representation using chemical equations
An example of a precipitation reaction: Aqueous silver nitrate (AgNO3) is added to a solution containing potassium chloride (KCl), the precipitation of a white solid, silver chloride (AgCl), is observed. (Zumdahl, 2005)
The silver chloride (AgCl) has formed a solid, which is observed as a precipitate.
This reaction can be written emphasizing the dissociated ions in a combined solution. This is known as the ionic equation.
A final way to represent a precipitate reaction is known as a net ionic reaction.
Precipitate colors

and Fe3+
.
Many compounds containing metal ions produce precipitates with distinctive colors. The following are typical colors for various metals. However, many of these compounds can produce colors very different from those listed.
| Gold | black |
| Chromium | Deep green, murky green, orange, purple, yellow, brown |
| Cobalt | Pink |
| Copper | Blue |
| Iron(II) | Green |
| Iron(III) | Reddish brown |
| Manganese | Pale pink |
| Nickel | Green |
| Lead | Yellow |
Other compounds generally form white precipitates.
Anion/cation analysis
Precipitate formation is useful in the detection of the type of cation in a salt. To do this, an alkali first reacts with the unknown salt to produce a precipitate that is the hydroxide of the unknown salt. To identify the cation, the color of the precipitate and its solubility in excess are noted. Similar processes are often used in sequence – for example, a barium nitrate solution will react with sulfate ions to form a solid barium sulfate precipitate, indicating that it is likely that sulfate ions are present.
Digestion
Digestion, or precipitate ageing, happens when a freshly formed precipitate is left, usually at a higher temperature, in the solution from which it precipitates. It results in cleaner and bigger particles. The physico-chemical process underlying digestion is called Ostwald ripening.
References
- Dhara, S. (2007). "Formation, Dynamics, and Characterization of Nanostructures by Ion Beam Irradiation". Critical Reviews in Solid State and Materials Sciences. 32 (1): 1–50. Bibcode:2007CRSSM..32....1D. doi:10.1080/10408430601187624.
- A. D. Adler; F. R. Longo; J. D. Finarelli; J. Goldmacher; J. Assour; L. Korsakoff (1967). "A simplified synthesis for meso-tetraphenylporphine". J. Org. Chem. 32 (2): 476–476. doi:10.1021/jo01288a053.
- Alan D. Adler; Frederick R. Longo; Frank Kampas; Jean Kim (1970). "On the preparation of metalloporphyrins". Journal of Inorganic and Nuclear Chemistry. 32 (7): 2443. doi:10.1016/0022-1902(70)80535-8.
- Dupont, J., Consorti, C., Suarez, P., de Souza, R. (2004). "Preparation of 1-Butyl-3-methyl imidazolium-based Room Temperature Ionic Liquids". Organic Syntheses.CS1 maint: multiple names: authors list (link); Collective Volume, 10, p. 184
Additional reading
- Zumdahl, Steven S. (2005). Chemical Principles (5th ed.). New York: Houghton Mifflin. ISBN 0-618-37206-7.
External links
| Wikimedia Commons has media related to Solid precipitation. |