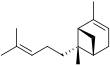
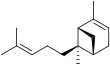
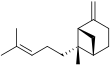
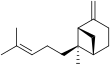
Bergamotene
Bergamotenes are a group of isomeric chemical compounds with the molecular formula C15H24. The bergamotenes are found in a variety of plants, particularly in their essential oils.
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
(α): 2,6-Dimethyl-6-(4-methylpent-3-enyl)bicyclo[3.1.1]hept-2-ene (β): 6-Methyl-2-methylidene-6-(4-methylpent-3-en-1-yl)bicyclo[3.1.1]heptane | |||
| Identifiers | |||
| |||
3D model (JSmol) |
| ||
PubChem CID |
|||
| UNII |
| ||
| |||
| Properties | |||
| C15H24 | |||
| Molar mass | 204.357 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
There are two structural isomers, α-bergamotene and β-bergamotene, which differ only by the location of a double bond. Both of these isomers have stereoisomers, the most common of which are known as the cis and trans-isomers (or endo- and exo-isomers).
α-Bergamotene is found in the oils of carrot, bergamot, lime, citron, cottonseed, and kumquat.[1][2]
Pheromones
The bergamotenes are pheromones for some insects. For example, β-trans-bergamotene is a pheromone for the wasp Melittobia digitata.[3] Plants can defend themselves against attack by herbivorous insects by producing pheromones such as bergamotenes that attract predators of those herbivores.[4][5] In a more complex relationship, the tobacco plant Nicotiana attenuata emits α-trans-bergamotene from its flowers at night to attract the tobacco hawk moth (Manduca sexta) as a pollinator; however, during the day the leaves produce α-trans-bergamotene to lure predatory insects to feed on any larvae and eggs that the pollinator may have produced.[6][7]
Biosynthesis
All the bergamotenes are biosynthesized from farnesyl pyrophosphate[8] via a variety of enzymes including exo-alpha-bergamotene synthase, (+)-endo-beta-bergamotene synthase, (-)-endo-alpha-bergamotene synthase, and others. Bergamotenes, in turn, are intermediates in the biosynthesis of more complex chemical compounds. For example, β-trans-bergamotene is a precursor in the biosynthesis of fumagillin, ovalicin, and related antibiotics.[8][9]
References
- "Metabocard for alpha-Bergamotene (HMDB0036678)". Human Metabolome Database.
- Koyasako, A.; Bernhard, R. A. (1983). "Volatile Constituents of the Essential Oil of Kumquat". Journal of Food Science. 48 (6): 1807–1812. doi:10.1111/j.1365-2621.1983.tb05090.x.
- "Semiochemical - beta-trans-bergamotene". pherobase.com.
- Kessler, A.; Baldwin, I. T. (2001). "Defensive Function of Herbivore-Induced Plant Volatile Emissions in Nature". Science. 291 (5511): 2141–2144. Bibcode:2001Sci...291.2141K. doi:10.1126/science.291.5511.2141. PMID 11251117.
- Schnee, C.; Kollner, T. G.; Held, M.; Turlings, T. C. J.; Gershenzon, J.; Degenhardt, J. (2006). "The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores". Proceedings of the National Academy of Sciences. 103 (4): 1129–1134. Bibcode:2006PNAS..103.1129S. doi:10.1073/pnas.0508027103. PMC 1347987. PMID 16418295.
- Zhou, Wenwu; Kügler, Anke; McGale, Erica; Haverkamp, Alexander; Knaden, Markus; Guo, Han; Beran, Franziska; Yon, Felipe; Li, Ran; Lackus, Nathalie; Köllner, Tobias G.; Bing, Julia; Schuman, Meredith C.; Hansson, Bill S.; Kessler, Danny; Baldwin, Ian T.; Xu, Shuqing (2017). "Tissue-Specific Emission of (E)-α-Bergamotene Helps Resolve the Dilemma when Pollinators Are Also Herbivores". Current Biology. 27 (9): 1336–1341. doi:10.1016/j.cub.2017.03.017. PMID 28434859.
- "Bergamotene—alluring and lethal for Manduca sexta". Max-Planck-Gesellschaft. April 24, 2017. Retrieved August 16, 2019.
- Cane, David E.; McIlwaine, Douglas B.; Harrison, Paul H. M. (1989). "Bergamotene biosynthesis and the enzymic cyclization of farnesyl pyrophosphate". Journal of the American Chemical Society. 111 (3): 1152–1153. doi:10.1021/ja00185a068.
- Cane, David E.; McIlwaine, Douglas B. (1987). "The biosynthesis of ovalicin from β-trans-bergamotene". Tetrahedron Letters. 28 (52): 6545–6548. doi:10.1016/S0040-4039(00)96909-0.