Triple-stranded DNA
Triple-stranded DNA (also known as H-DNA or Triplex-DNA) is a DNA structure in which three oligonucleotides wind around each other and form a triple helix. In triple-stranded DNA, the third strand binds to a B-form DNA (via Watson–Crick base-pairing) double helix by forming Hoogsteen base pairs or reversed Hoogsteen hydrogen bonds.
Structures

Hoogesteen base pairing
A thymine (T) nucleobase can bind to a Watson–Crick base-pairing of T-A by forming a Hoogsteen hydrogen bond. The thymine hydrogen bonds with the adenosine (A) of the original double-stranded DNA to create a T-A*T base-triplet.[1] Under acidic conditions, a protonated cytosine, represented as C+, can form a base-triplet with a C-G pair through Hoogsteen base-pairing, forming C-G*C+. The TA*T and CG*C+ base pairs are the most stabilized triplet-base pairs that can form, while a TA*G and CG*G are the most destabilized triplet-base pairs.[2]
Intermolecular and intramolecular formations
There are two classes of triplex DNA: intermolecular and intramolecular formations. An intermolecular triplex refers to triplex formation between a duplex and a different (third) strand of DNA. The third strand can either be from a neighboring chromosome or a triplex forming oligonucleotide (TFO). Intramolecular triplex DNA is formed from a duplex with homopurine and homopyrimidine strands with mirror repeat symmetry.[3] The degree of supercoiling in DNA influences the amount of intramolecular triplex formation that occurs.[4] There are two different types of intramolecular triplex DNA: H-DNA and H*-DNA. Formation of H-DNA is stabilized under acidic conditions and in the presence of divalent cations such as Mg2+. In this conformation, the homopyrimidine strand in the duplex bends back to bind to the purine strand in a parallel fashion. The base triads used to stabilize this conformation are T-A*T and C-G*C+. The cytosine of this base triad needs to be protonated in order to form this intramolecular triple helix, which is why this conformation is stabilized under acidic conditions.[5] H*-DNA has favorable formation conditions at neutral pH and in the presence of divalent cations.[4] This intramolecular conformation is formed from the binding of the homopurine and purine strand of the duplex in an antiparallel fashion. It is stabilized by T-A*A and C-G*G base triplets.[3][5]
Function
Triple-stranded DNA has been implicated in the regulation of several genes. For instance, the c-myc gene has been extensively mutated to examine the role that triplex DNA, versus the linear sequence, plays in gene regulation. A c-myc promoter element, termed the nuclease-sensitive element or NSE, can form tandem intramolecular triplexes of the H-DNA type and has a repetitive sequence motif (ACCCTCCCC)4. The mutated NSE was examined for transcriptional activity and for its intra- and intermolecular triplex-forming ability. The transcriptional activity of mutant NSEs can be predicted by the element's ability to form H-DNA and not by repeat number, position, or the number of mutant base pairs. DNA may therefore be a dynamic participant in the transcription of the c-myc gene.[6]
Triplex Forming Oligonucleotides (TFO)
TFOs are short (≈15-25 nt) nucleic acid strands that bind in the major groove of double-stranded DNA to form intramolecular triplex DNA structures. There is some evidence that they are also able to modulate gene activity in vivo. In peptide nucleic acid (PNA), the sugar-phosphate backbone of DNA is replaced with a protein-like backbone. PNAs form P-loops while interacting with duplex DNA, forming a triplex with one strand of DNA while displacing the other. Very unusual recombination or parallel triplexes, or R-DNA, have been assumed to form under RecA protein in the course of homologous recombination.[7]
TFOs bind specifically to homopurine-homopyrimidine regions that are often common in promoter and intron sequences of genes, influencing cell signaling.[8] TFOs can inhibit transcription by binding with high specificity to the DNA helix, thereby blocking the binding and function of transcription factors for particular sequences. By introducing TFOs into a cell (through transfection or other means), the expression of certain genes can be controlled.[9] This application has novel implications in site-specific mutagenesis and gene therapy. In human prostate cancer cells, a transcription factor Ets2 is over-expressed and thought to drive forward the growth and survival of cells in such excess. Carbone et al. designed a sequence-specific TFO to the Ets2 promoter sequence that down-regulated the gene expression and led to a slowing of cell growth and cell death.[10] Changxian et al. have also presented a TFO targeting the promoter sequence of bcl-2, a gene inhibiting apoptosis.[11]
The observed inhibition of transcription can also have negative health effects like its role in the recessive, autosomal gene for Friedreich’s Ataxia.[12] In Fredrick’s Ataxia, triplex DNA formation impairs the expression of intron 1 of the FXN gene. This results in the degeneration of the nervous system and spinal cord, impairing the movement of the limbs.[13] To combat this triplex instability, nucleotide excision repair proteins (NERs) have been shown to recognize and repair triple-stranded DNA structures, reinstating full availability of the previously inhibited and unstable gene.[14]
Genetic instability
Considerable research has been funneled into the biological implications relating to the presence of triplex DNA, more specifically H-DNA, in the major breakpoint regions (Mbr) and double-strand-breakpoints (DBS) of certain genes.
For example, in addition to other non-B DNA sequences found neighboring the P1 promoter of the c-MYC gene, polypurine mirror-repeat H-DNA forming sequences were found and are associated with the major breakpoint hotspots of this region. Cases of genetic instability were also observed in the F1 offspring of transgenic mice after incorporation of human H-DNA-forming sequences paired with Z-DNA sequences into their genomes where no instability was previously reported.[15] Additionally, formation of R.R.Y. triplex conformations have been observed at the Mbr of the bcl-2 gene. Formation of these structures has been posited to cause the t(14;18) translocation observed in many cancers and most follicular lymphomas. This observation has led to research that indicated a substantial decrease in translocation events can be observed after blocking the formation of H-DNA by altering the sequence of this region slightly.[15][16] Long tracts of GAA·TTC have also been observed to form very stable triplex structures. Interactions between these two triplex structures, termed sticky DNA, has been shown to interrupt transcription of the X25, or frataxin gene. As decreased levels of the protein frataxin is associated with Friedreich's ataxia, formation of this instability has been suggested to be the basis for this genetic disease.[17][18]
History
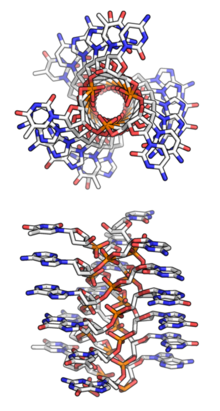
Triple-stranded DNA structures were common hypotheses in the 1950s when scientists were struggling to discover DNA's true structural form. Watson and Crick (who later won the Nobel Prize for their double-helix model) originally considered a triple-helix model, as did Pauling and Corey, who published a proposal for their triple-helix model in 1953,[19][20] as well as fellow scientist Fraser.[21] However, Watson and Crick soon identified several problems with these models:
- Negatively charged phosphates near the axis repel each other, leaving the question of how the three-chain structure stays together.
- In a triple-helix model (specifically Pauling and Corey's model), some of the van der Waals distances appear to be too small.
Fraser's model differed from Pauling and Corey's in that in his model the phosphates are on the outside and the bases are on the inside, linked together by hydrogen bonds. However, Watson and Crick found Fraser's model to be too ill-defined to comment specifically on its inadequacies.
An alternative triple-stranded DNA structure was described in 1957.[22] It was thought to occur in only one in vivo biological process: as an intermediate product during the action of the E. coli recombination enzyme RecA. Its role in that process is not understood.[22]
References
- Rhee S, Han Z, Liu K, Miles HT, Davies DR (December 1999). "Structure of a triple helical DNA with a triplex-duplex junction". Biochemistry. 38 (51): 16810–5. doi:10.1021/bi991811m. PMID 10606513.
- Mergny JL, Sun JS, Rougée M, Montenay-Garestier T, Barcelo F, Chomilier J, Hélène C (October 1991). "Sequence specificity in triple-helix formation: experimental and theoretical studies of the effect of mismatches on triplex stability". Biochemistry. 30 (40): 9791–8. doi:10.1021/bi00104a031. PMID 1911764.
- Ussery DW, Sinden RR (June 1993). "Environmental influences on the in vivo level of intramolecular triplex DNA in Escherichia coli". Biochemistry. 32 (24): 6206–13. doi:10.1021/bi00075a013. PMID 8512930.
- Dayn A, Samadashwily GM, Mirkin SM (December 1992). "Intramolecular DNA triplexes: unusual sequence requirements and influence on DNA polymerization". Proceedings of the National Academy of Sciences of the United States of America. 89 (23): 11406–10. Bibcode:1992PNAS...8911406D. doi:10.1073/pnas.89.23.11406. PMC 50559. PMID 1454828.
- Lyamichev VI, Mirkin SM, Frank-Kamenetskii MD (February 1986). "Structures of homopurine-homopyrimidine tract in superhelical DNA". Journal of Biomolecular Structure & Dynamics. 3 (4): 667–9. doi:10.1080/07391102.1986.10508454. PMID 3271043.
- Firulli AB, Maibenco DC, Kinniburgh AJ (April 1994). "Triplex forming ability of a c-myc promoter element predicts promoter strength". Archives of Biochemistry and Biophysics. 310 (1): 236–42. doi:10.1006/abbi.1994.1162. PMID 8161210.
- Frank-Kamenetskii MD, Mirkin SM (1995-01-01). "Triplex DNA structures". Annual Review of Biochemistry. 64: 65–95. doi:10.1146/annurev.bi.64.070195.000433. PMID 7574496.
- Brázdová M, Tichý V, Helma R, Bažantová P, Polášková A, Krejčí A, et al. (2016). "p53 Specifically Binds Triplex DNA In Vitro and in Cells". PLOS One. 11 (12): e0167439. doi:10.1371/journal.pone.0167439. PMC 5131957. PMID 27907175.
- Graham MK, Brown TR, Miller PS (Apr 2015). "Targeting the human androgen receptor gene with platinated triplex-forming oligonucleotides". Biochemistry. 54 (13): 2270–82. doi:10.1021/bi501565n. PMID 25768916.
- Carbone GM, Napoli S, Valentini A, Cavalli F, Watson DK, Catapano CV (2004-08-03). "Triplex DNA-mediated downregulation of Ets2 expression results in growth inhibition and apoptosis in human prostate cancer cells". Nucleic Acids Research. 32 (14): 4358–67. doi:10.1093/nar/gkh744. PMC 514370. PMID 15314206.
- Shen C, Rattat D, Buck A, Mehrke G, Polat B, Ribbert H, Schirrmeister H, Mahren B, Matuschek C, Reske SN (February 2003). "Targeting bcl-2 by triplex-forming oligonucleotide--a promising carrier for gene-radiotherapy". Cancer Biotherapy & Radiopharmaceuticals. 18 (1): 17–26. doi:10.1089/108497803321269296. PMID 12667305.
- Sakamoto N, Chastain PD, Parniewski P, Ohshima K, Pandolfo M, Griffith JD, Wells RD (April 1999). "Sticky DNA: self-association properties of long GAA.TTC repeats in R.R.Y triplex structures from Friedreich's ataxia". Molecular Cell. 3 (4): 465–75. doi:10.1016/s1097-2765(00)80474-8. PMID 10230399.
- Bacolla A, Wells RD (April 2009). "Non-B DNA conformations as determinants of mutagenesis and human disease". Molecular Carcinogenesis. 48 (4): 273–85. doi:10.1002/mc.20507. PMID 19306308.
- Kaushik Tiwari M, Adaku N, Peart N, Rogers FA (September 2016). "Triplex structures induce DNA double strand breaks via replication fork collapse in NER deficient cells". Nucleic Acids Research. 44 (16): 7742–54. doi:10.1093/nar/gkw515. PMC 5027492. PMID 27298253.
- Wang G, Vasquez KM (July 2014). "Impact of alternative DNA structures on DNA damage, DNA repair, and genetic instability". DNA Repair. 19: 143–51. doi:10.1016/j.dnarep.2014.03.017. PMC 4216180. PMID 24767258.
- Raghavan SC, Chastain P, Lee JS, Hegde BG, Houston S, Langen R, Hsieh CL, Haworth IS, Lieber MR (June 2005). "Evidence for a triplex DNA conformation at the bcl-2 major breakpoint region of the t(14;18) translocation". The Journal of Biological Chemistry. 280 (24): 22749–60. doi:10.1074/jbc.M502952200. PMID 15840562.
- Wang G, Vasquez KM (September 2004). "Naturally occurring H-DNA-forming sequences are mutagenic in mammalian cells". Proceedings of the National Academy of Sciences of the United States of America. 101 (37): 13448–53. doi:10.1073/pnas.0405116101. PMC 518777. PMID 15342911.
- Vetcher AA, Napierala M, Iyer RR, Chastain PD, Griffith JD, Wells RD (October 2002). "Sticky DNA, a long GAA.GAA.TTC triplex that is formed intramolecularly, in the sequence of intron 1 of the frataxin gene". The Journal of Biological Chemistry. 277 (42): 39217–27. doi:10.1074/jbc.M205209200. PMID 12161437.
- Pauling L, Corey RB (February 1953). "A Proposed Structure For The Nucleic Acids". Proceedings of the National Academy of Sciences of the United States of America. 39 (2): 84–97. doi:10.1073/pnas.39.2.84. PMC 1063734. PMID 16578429.
- Pauling L, Corey RB (February 1953). "Structure of the nucleic acids". Nature. 171 (4347): 346. doi:10.1038/171346a0. PMID 13036888.
- Fraser B (1953). "The Structure of Deoxyribose Nucleic Acid". Journal of Structural Biology. 145 (3): 184–5. doi:10.1016/j.jsb.2004.01.001. PMID 14997898.
- Felsenfeld G, Davies DR, Rich A (1957). "Formation of a three-stranded polynucleotide molecule". Journal of the American Chemical Society. 79 (8): 2023–2024. doi:10.1021/ja01565a074.
Further reading
- Rich A (December 1993). "DNA comes in many forms". Gene. 135 (1–2): 99–109. doi:10.1016/0378-1119(93)90054-7. PMID 8276285.
- Soyfer VN, Potaman VN (1995). Triple-Helical Nucleic Acids. New York: Springer. ISBN 978-0-387-94495-1.
- Mills M, Arimondo PB, Lacroix L, Garestier T, Hélène C, Klump H, Mergny JL (September 1999). "Energetics of strand-displacement reactions in triple helices: a spectroscopic study". Journal of Molecular Biology. 291 (5): 1035–54. doi:10.1006/jmbi.1999.3014. PMID 10518941.
- Watson JD, Crick FH (April 1953). "Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid". Nature. 171 (4356): 737–8. Bibcode:1953Natur.171..737W. doi:10.1038/171737a0. PMID 13054692.
.png)