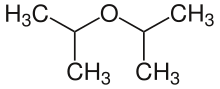
Diisopropyl ether
Diisopropyl ether is secondary ether that is used as a solvent. It is a colorless liquid that is slightly soluble in water, but miscible with organic solvents. It is used as an extractant and an oxygenate gasoline additive. It is obtained industrially as a byproduct in the production of isopropanol by hydration of propene.[3] Diisopropyl ether is sometimes represented by the abbreviation "DIPE".
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-[(Propan-2-yl)oxy]propane | |
| Other names
Isopropyl ether 2-Isopropoxypropane Diisopropyl oxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.237 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1159 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H14O | |
| Molar mass | 102.177 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Sharp, sweet, ether-like[1] |
| Density | 0.725 g/ml |
| Melting point | −60 °C (−76 °F; 213 K) |
| Boiling point | 68.5 °C (155.3 °F; 341.6 K) |
| 2 g/L at 20 °C | |
| Vapor pressure | 119 mmHg (20°C)[1] |
| -79.4·10−6 cm3/mol | |
| Hazards | |
| GHS pictograms |    |
| GHS Signal word | Danger |
GHS hazard statements |
H225, H316, H319, H335, H336, H361, H371, H402, H412 |
| P201, P202, P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P281, P303+361+353, P304+340, P305+351+338, P308+313, P309+311, P312, P332+313, P337+313, P370+378 | |
| NFPA 704 (fire diamond) | |
| Flash point | −28 °C (−18 °F; 245 K) |
| 443 °C (829 °F; 716 K) | |
| Explosive limits | 1.4–7.9% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
8470 mg/kg (rat, oral)[2] |
LDLo (lowest published) |
5000-6500 mg/kg (rabbit, oral)[2] |
LC50 (median concentration) |
38,138 ppm (rat) 30,840 ppm (rabbit) 28,486 ppm (rabbit)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 500 ppm (2100 mg/m3)[1] |
REL (Recommended) |
TWA 500 ppm (2100 mg/m3)[1] |
IDLH (Immediate danger) |
1400 ppm[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
Whereas at 20 °C, diethyl ether will dissolve 1% by weight water, DIPE only dissolves 0.88%. It is used as a specialized solvent to remove or extract polar organic compounds from aqueous solutions, e.g. phenols, ethanol, acetic acid. DIPE was used as an antiknock agent.
Safety
Diisopropyl ether can form explosive peroxides upon standing in air for long periods. This reaction proceeds more easily than for ethyl ether, due to the secondary carbon next to the oxygen atom. Antioxidants can be used to prevent this process. The stored solvent should therefore be tested for the presence of peroxides more often (recommended once every 3 months for diisopropyl ether vs. once every 12 months for ethyl ether[4]). Peroxides may be removed by shaking the ether with an aqueous solution of iron(II) sulfate or sodium metabisulfite.[5][6] For safety reasons, methyl tert-butyl ether is often used as an alternative solvent.
See also
- Dimethyl ether
- Diethyl ether
- Dipropyl ether
- Di-tert-butyl ether
- Methyl tert-butyl ether
References
- NIOSH Pocket Guide to Chemical Hazards. "#0362". National Institute for Occupational Safety and Health (NIOSH).
- "Isopropyl ether". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Sakuth, Michael; Mensing, Thomas; Schuler, Joachim; Heitmann, Wilhelm; Strehlke, Günther; Mayer (2010). "Ethers, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_023.pub2.
- http://www.ccohs.ca/oshanswers/chemicals/organic/organic_peroxide.html
- Chai, Christina Li Lin; Armarego, W. L. F. (2003). Purification of laboratory chemicals. Oxford: Butterworth-Heinemann. p. 176. ISBN 978-0-7506-7571-0.
- Hamstead, A. C. (1964). "Destroying Peroxides of Isopropyl Ether". Industrial and Engineering Chemistry. 56: 37-42. doi:10.1021/ie50654a005.
