Clathrate compound
A clathrate is a chemical substance consisting of a lattice that traps or contains molecules. The word clathrate is derived from the Latin clathratus (clatratus), meaning ‘with bars, latticed’.[1] Traditionally, clathrate compounds are polymeric and completely envelop the guest molecule, but in modern usage clathrates also include host–guest complexes and inclusion compounds.[2] According to IUPAC, clathrates are inclusion compounds "in which the guest molecule is in a cage formed by the host molecule or by a lattice of host molecules."[3]
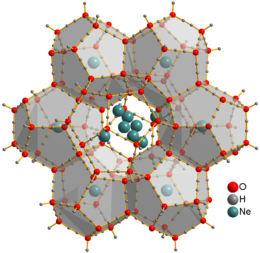
Occurrence and scope
Traditionally clathrate compounds refer to polymeric hosts containing molecular guests. More recently, the term refers to many molecular hosts, including calixarenes and cyclodextrins and even some inorganic polymers such as zeolites. The natural silica clathrate mineral, chibaite was described from Japan in 2008.
Many clathrates are derived from organic hydrogen-bonded frameworks. These frameworks are prepared from molecules that "self-associate" by multiple hydrogen-bonding interactions. The most famous clathrates are methane clathrates where the hydrogen-bonded framework is contributed by water and the guest molecules are methane. Large amounts of methane naturally frozen in this form exist both in permafrost formations and under the ocean sea-bed.[4] Other hydrogen-bonded networks are derived from hydroquinone, urea, and thiourea. A much studied host molecule is Dianin's compound.
2CCl4.jpg)
Hofmann compounds are coordination polymers with the formula Ni(CN)4·Ni(NH3)2. These materials crystallize with small aromatic guests (benzene, certain xylenes), and this selectivity has been exploited commercially for the separation of these hydrocarbons.[2] Metal organic frameworks (MOFs) form clathrates.

Photolytically-sensitive caged compounds have been examined as containers for releasing a drug or reagent.[5]
History
Clathrate hydrates were discovered in 1810 by Humphry Davy.[6] Clathrates were studied by P. Pfeiffer in 1927 and in 1930, E. Hertel defined "molecular compounds" as substances decomposed into individual components following the mass action law in solution or gas state. In 1945, H. M. Powell analyzed the crystal structure of these compounds and named them clathrates.
Related materials
Inclusion compounds are often molecules, whereas clathrates are typically polymeric. Intercalation compounds are not 3-dimensional, unlike clathrate compounds.
See also
References
- Latin dictionary Archived 2012-04-14 at the Wayback Machine
- Atwood, J. L. (2012) "Inclusion Compounds" in Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim. doi: 10.1002/14356007.a14_119
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "clathrates". doi:10.1351/goldbook.C01097
- Pearce, Fred (27 June 2009). "Ice on fire: The next fossil fuel". New Scientist (2714). pp. 30–33. Archived from the original on April 13, 2016. Retrieved July 5, 2009.
- Ellis-Davies, Graham C. R. (2007). "Caged compounds: Photorelease technology for control of cellular chemistry and physiology". Nature Methods. 4 (8): 619–628. doi:10.1038/nmeth1072. PMC 4207253. PMID 17664946.
- Thomas, Ellen (November 2004). "Clathrates: little known components of the global carbon cycle". Wesleyan University. Retrieved 13 December 2007.
| Wikimedia Commons has media related to Clathrates. |