4-Quinolone
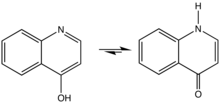
4-Quinolone is an organic compound derived from quinoline. It and 2-quinolone are the two most important parent (meaning simplified) quinolones. 4-Quinolone exists in equilibrium with a minor tautomer, 4-hydroxyquinoline (CAS#611-36-9). Aside from pedagogical interest, 4-Quinolone is of little intrinsic value but its derivatives, the 4-quinolone antibiotics represent a large class of important drugs.[1]
 | |
| Names | |
|---|---|
| Other names
1H-Quinolin-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ECHA InfoCard | 100.009.336 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H7NO | |
| Molar mass | 145.161 g·mol−1 |
| Melting point | 208–210 °C (406–410 °F; 481–483 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
The chemical synthesis of quinolones often involves ring-closing reactions.[2] Such reactions often install a hydroxyl group (an –OH functional group) on the carbon across from the ring nitrogen (i.e., the C-4 positions). An example of such a synthesis is the Camps cyclization, which, depending on starting materials and reaction conditions, can give both 2-hydroxyquinolines (B) and 4-hydroxyquinolines (A) as shown. The hydroxyquinolines tautomerize to the quinolones.

References
- Andriole, VT The Quinolones. Academic Press, 1989.
- Shi, Pengfei; Wang, Lili; Chen, Kehao; Wang, Jie; Zhu, Jin (2017). "Co(III)-Catalyzed Enaminone-Directed C-H Amidation for Quinolone Synthesis". Organic Letters. 19: 2418–2421. doi:10.1021/acs.orglett.7b00968. PMID 28425721.