2-Quinolone
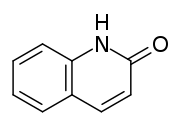
2-Quinolone is an organic compound related structurally to quinoline. It is the majority tautomer in equilibrium with 2-quinolinol. The compound can be classified as a cyclic amide, and as such is used as an isostere for peptides and other pharmaceutically-inspired targets.[1] The isomer 4-quinolone is the parent of a large class of quinolone antibiotics.

2-Quinolone (right) and its tautomer 2-hydroxylquinoline (left)
 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.382 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H7NO | |
| Molar mass | 145.161 g·mol−1 |
| Appearance | solid |
| Melting point | 199.5 °C (391.1 °F; 472.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
One example is Ravesilone.
References
- Tashima, Toshihiko (2015). "The structural use of carbostyril in physiologically active substances". Bioorganic & Medicinal Chemistry Letters. 25: 3415–3419. doi:10.1016/j.bmcl.2015.06.027. PMID 26112444.
External links

This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.