You can't get black
While it is possible to achieve different ranges of colours depending on temperature of the flame as well as combustible material, fire's still light.
Light is bound to the spectrum of visible wavelengths. And there is no visible wavelength for black.
Addition1: It is important to understand that the light of fire is additive. Hence if you'd want to go towards black fire, you'd actually have to find a way to remove light-sources from a) the fire itself and b) its surroundings; thus creating a space that absorbs light.
But what colours of fire can we have?
Looking at pyrotechnics we can achieve the following colours by e.g. burning different metal salts:
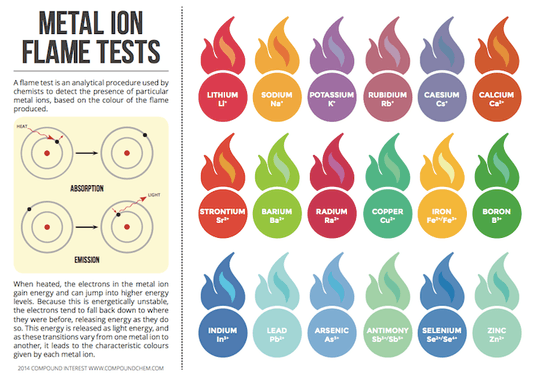 Chart of what substance creates which flame colour - found on http://www.compoundchem.com
Chart of what substance creates which flame colour - found on http://www.compoundchem.com
These colours are achieved, as already stated, by burning salts. As long as we know where to find metals, we can dig them up and refine most of them with as little tools as a vat of acid and some patience.
The most accessible of these would probably be:
- Lithium
- Potassium
- Calcium
- Copper
- Iron
- Lead
- Zinc
1Thank you Slipp D. Thompson

1
This is the exact basis of atomic emission spectroscopy...https://en.wikipedia.org/wiki/Atomic_emission_spectroscopy
– DJohnM – 2017-06-01T21:52:12.0433I just hope he has enough money to buy the materials to change the colour of the flames. Also its probably not the greatest idea to eat the poisonous fire, eat the normal one and do other tricks with the different coloured flames. – Necessity – 2017-06-02T00:24:37.967
or just don't use poisonous ones – Foxy – 2017-06-02T00:25:33.490
1This is also how the colors of fireworks are produced. Fireworks in general were invented in ~7th century CE China; I don't know exactly how old colored fireworks are, but knowing that certain substances burn colorfully is totally plausible for "medieval". – zwol – 2017-06-02T01:45:25.043
You ought to wait at least 24 hours before “accepting” an answer here on WB. Also, please don’t change the question once it has been answered! Finally, would you please type your capital letters and punctuation marks?! All of your questions have been “fixed” by others to appear normal. – JDługosz – 2017-06-02T08:57:00.580
1In the process of entertaining people try to not get burned for witchery. – BlueWizard – 2017-06-06T21:32:47.610