Xylene
Xylene (from Greek ξύλο, xylo, "wood"), xylol or dimethylbenzene is any one of three isomers of dimethylbenzene, or a combination thereof. With the formula (CH3)2C6H4, each of the three compounds has a central benzene ring with two methyl groups attached at substituents. They are all colorless, flammable liquids, some of which are of great industrial value. The mixture is referred to as both xylene and, more precisely, xylenes.
Occurrence and production
Xylenes are an important petrochemical produced by catalytic reforming and also by coal carbonisation in the manufacture of coke fuel. They also occur in crude oil in concentrations of about 0.5–1%, depending on the source. Small quantities occur in gasoline and aircraft fuels.
Xylenes are produced mainly as part of the BTX aromatics (benzene, toluene, and xylenes) extracted from the product of catalytic reforming known as reformate. The xylene mixture is a slightly greasy, colorless liquid commonly encountered as a solvent.
Several million tons are produced annually.[1] In 2011, a global consortium began construction of one of the world's largest xylene plants in Singapore.[2]
History
Xylene was first isolated and named in 1850 by the French chemist Auguste Cahours (1813–1891), having been discovered as a constituent of wood tar.[3]
Isomers
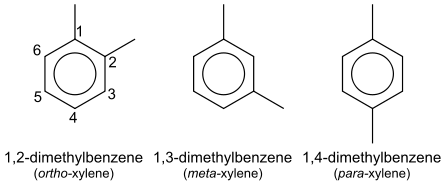
Xylene exists in three isomeric forms. The isomers can be distinguished by the designations ortho- (o-), meta- (m-) and para- (p-), which specify to which carbon atoms (of the benzene ring) the two methyl groups are attached. By counting the carbon atoms around the ring starting from one of the ring carbons bonded to a methyl group, and counting towards the second methyl group, the o-isomer has the IUPAC name of 1,2-dimethylbenzene, the m-isomer is 1,3-dimethylbenzene and the p-isomer is 1,4-dimethylbenzene. Of the three isomers, the p-isomer is the most industrially sought after since it can be oxidized to terephthalic acid.[1]
Industrial production
Xylenes are produced by the methylation of toluene and benzene.[1][4] Commercial or laboratory grade xylene produced usually contains about 40-65% of m-xylene and up to 20% each of o-xylene, p-xylene and ethylbenzene.[5][6][7] The ratio of isomers can be shifted to favor the highly valued p-xylene via the patented UOP-Isomar process[8] or by transalkylation of xylene with itself or trimethylbenzene. These conversions are catalyzed by zeolites.[1]
ZSM-5 is used to facilitate some isomerization reactions leading to mass production of modern plastics.
Properties
The chemical and physical properties of xylene differ according to the respective isomers. The melting point ranges from −47.87 °C (−54.17 °F) (m-xylene) to 13.26 °C (55.87 °F) (p-xylene)—as usual, the para isomer's melting point is much higher because it packs more readily in the crystal structure. The boiling point for each isomer is around 140 °C (284 °F). The density of each isomer is around 0.87 g/mL (7.26 lb/U.S. gallon or 8.72 lb/imp gallon) and thus is less dense than water. Xylene in air can be smelled at concentrations as low as 0.08 to 3.7 ppm (parts of xylene per million parts of air) and can be tasted in water at 0.53 to 1.8 ppm.[6]
| Xylene isomers | ||||
|---|---|---|---|---|
| General | ||||
| Common name | Xylene | o-Xylene | m-Xylene | p-Xylene |
| Systematic name | Dimethylbenzene | 1,2-Dimethylbenzene | 1,3-Dimethylbenzene | 1, 4-Dimethylbenzene |
| Other names | Xylol | o-Xylol; Orthoxylene |
m-Xylol; Metaxylene |
p-Xylol; Paraxylene |
| Molecular formula | C8H10 | |||
| SMILES | Cc1c(C)cccc1 | Cc1cc(C)ccc1 | Cc1ccc(C)cc1 | |
| Molar mass | 106.16 g/mol | |||
| Appearance | Clear, colorless liquid | |||
| CAS number | [1330-20-7] | [95-47-6] | [108-38-3] | [106-42-3] |
| Properties | ||||
| Density and phase | 0.864 g/mL, liquid | 0.88 g/mL, liquid | 0.86 g/mL, liquid | 0.86 g/mL, liquid |
| Solubility in water | Practically insoluble | |||
| Soluble in non-polar solvents such as aromatic hydrocarbons | ||||
| Melting point | −47.4 °C (−53.3 °F; 226 K) | −25 °C (−13 °F; 248 K) | −48 °C (−54 °F; 225 K) | 13 °C (55 °F; 286 K) |
| Boiling point | 138.5 °C (281.3 °F; 412 K) | 144 °C (291 °F; 417 K) | 139 °C (282 °F; 412 K) | 138 °C (280 °F; 411 K) |
| Viscosity | 0.812 cP at 20 °C (68 °F) | 0.62 cP at 20 °C (68 °F) | 0.34 cP at 30 °C (86 °F) | |
| Hazards | ||||
| SDS | Xylenes[9] | o-Xylene | m-Xylene | p-Xylene |
| EU classification | Harmful (Xn) | |||
| NFPA 704 | ||||
| Flash point | 30 °C (86 °F) | 17 °C (63 °F) | 25 °C (77 °F) | 25 °C (77 °F) |
| R/S statement | R10, R20/21, R38: (S2), S25 | |||
| RTECS number | ZE2450000 | ZE2275000 | ZE2625000 | |
| Related compounds | ||||
| Related aromatic hydrocarbons |
Toluene, mesitylene, benzene, ethylbenzene | |||
| Related compounds | Xylenols - types of phenols | |||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | ||||
Xylenes form azeotropes with water and a variety of alcohols. With water the azeotrope consists of 60% xylenes and boils at 94.5 °C.[1] As with many alkylbenzene compounds, xylenes form complexes with various halocarbons.[10] The complexes of different isomers often have dramatically different properties from each other.[11]
Applications
Terephthalic acid and related derivatives
p-Xylene is the principal precursor to terephthalic acid and dimethyl terephthalate, both monomers used in the production of polyethylene terephthalate (PET) plastic bottles and polyester clothing. 98% of p-xylene production, and half of all xylenes produced is consumed in this manner.[7][12] o-Xylene is an important precursor to phthalic anhydride. The demand for isophthalic acid is relatively modest so m-xylene is rarely sought (and hence the utility of its conversion to the o- and p-isomers).
Solvent applications and industrial purposes
Xylene is used as a solvent. In this application, with a mixture of isomers, it is often referred to as xylenes or xylol. Solvent xylene often contains a small percentage of ethylbenzene. Like the individual isomers, the mixture is colorless, sweet-smelling, and highly flammable. Areas of application include the printing, rubber, and leather industries. It is a common component of ink, rubber, and adhesives.[13] In thinning paints and varnishes, it can be substituted for toluene where slower drying is desired, and thus is used by conservators of art objects in solubility testing.[14] Similarly it is a cleaning agent, e.g., for steel, silicon wafers, and integrated circuits. In dentistry, xylene can be used to dissolve gutta percha, a material used for endodontics (root canal treatments). In the petroleum industry, xylene is also a frequent component of paraffin solvents, used when the tubing becomes clogged with paraffin wax. For similar reasons, it is often the active ingredient in commercial products for ear wax (cerumen) removal.(1)
Laboratory use
Xylene is used in the laboratory to make baths with dry ice to cool reaction vessels,[15] and as a solvent to remove synthetic immersion oil from the microscope objective in light microscopy.[16] In histology, xylene is the most widely used clearing agent.[17] Xylene is used to remove paraffin from dried microscope slides prior to staining. After staining, microscope slides are put in xylene prior to mounting with a coverslip.
Precursor to other compounds
Although conversion to terephthalic acid is the dominant chemical conversion, xylenes are precursors to other chemical compounds. For instance chlorination of both methyl groups gives the corresponding xylene dichlorides (bis(chloromethyl)benzenes) whilst mono-bromination yields xylyl bromide, a tear gas agent used in World War I.
Health and safety
Xylene is flammable but of modest acute toxicity, with LD50 ranges from 200 to 5000 mg/kg for animals. Oral LD50 for rats is 4300 mg/kg. The principal mechanism of detoxification is oxidation to methylbenzoic acid and hydroxylation to hydroxylene.[1]
The main effect of inhaling xylene vapor is depression of the central nervous system (CNS), with symptoms such as headache, dizziness, nausea and vomiting. At an exposure of 100 ppm, one may experience nausea or a headache. At an exposure between 200 and 500 ppm, symptoms can include feeling "high", dizziness, weakness, irritability, vomiting, and slowed reaction time.[18][19]
The side effects of exposure to low concentrations of xylene (< 200 ppm) are reversible and do not cause permanent damage. Long-term exposure may lead to headaches, irritability, depression, insomnia, agitation, extreme tiredness, tremors, hearing loss, impaired concentration and short-term memory loss.[20] A condition called chronic solvent-induced encephalopathy, commonly known as "organic solvent syndrome" has been associated with xylene exposure. There is very little information available that isolates xylene from other solvent exposures in the examination of these effects.[18]
Hearing disorders have been also linked to xylene exposure, both from studies with experimental animals,[21][22] as well as clinical studies.[23][24][25]
Xylene is also a skin irritant and strips the skin of its oils, making it more permeable to other chemicals. The use of impervious gloves and masks, along with respirators where appropriate, is recommended to avoid occupational health issues from xylene exposure.[18]
Xylenes are metabolized to methylhippuric acids.[26][27] The presence of methylhippuric acid can be used as a biomarker to determine exposure to xylene.[27][28]
References
- Fabri, Jörg; Graeser, Ulrich; Simo, Thomas A. (2000). "Xylenes". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a28_433. ISBN 978-3527306732.
- Tremblay, Jean-François (2011). "Making Aromatics in Singapore". Chemical & Engineering News Archive. 89 (38): 18–19. doi:10.1021/cen-v089n038.p018.
- Cahours, Auguste (1850) "Recherches sur les huiles légéres obtenues dans la distillation du bois" (Investigations of light oils obtained by the distillation of wood), Compte rendus, 30 : 319-323 ; see especially p. 321. From p. 321: "Je le désignerai sous le nomme xylène." (I will designate it by the name of xylene.) Note: Cahours' empirical formula for xylene is incorrect because chemists at that time used the wrong atomic mass for carbon (6 instead of 12).
- Martindale, David C. and Kuchar, Paul J., Production of xylenes from light aliphatic hydrocarbons via dehydrocyclodimerization and methylation, United States Patent No. 5,043,502, 1991-8-27. Accessed 2012-4-28.
- "Xylene (Mixed Isomers), Air Toxic Hazard Summary)". United States Environmental Protection Agency. Retrieved 8 February 2015.
- Kandyala, Reena; Raghavendra, Sumanth Phani C.; Rajasekharan, Saraswathi T. (2010). "Xylene: An overview of its health hazards and preventive measures". J Oral Maxillofac Pathol. 14 (1): 1–5. doi:10.4103/0973-029X.64299. PMC 2996004. PMID 21180450.
- Xylene Archived August 11, 2011, at the Wayback Machine, Swedish Chemicals Agency, apps.kemi.se, 2010. Accessed 2012-4-28.
- "Capturing Opportunities for Para-xylene Production". UOP, A Honeywell Company. Retrieved 8 February 2015.
- SIRI, Xylenes Materials Safety Data Sheet, MSDS No. X2000, Vermont Safety Information Resources, Inc., 1997-9-8. Accessed 2012-4-27.
- Clark J. E.; Luthy, R. V. (1955). "Separation of Xylenes". Ind. Eng. Chem. 47 (2): 250–253. doi:10.1021/ie50542a028.
- Stevenson, Cheryl D; McElheny, Daniel J; Kage, David E; Ciszewski, James T; Reiter, Richard C (1998). "Separation of Closely Boiling Isomers and Identically Boiling Isotopomers via Electron-Transfer-Assisted Extraction". Analytical Chemistry. 70 (18): 3880. doi:10.1021/ac980221b.
- ICIS, Paraxylene-Orthoxylene | Prices, News & Market Analysis, icis.com, 2012. Accessed 2012-4-28.
- Bostik, Safety Data Sheet Blu-Tack Archived September 11, 2011, at the Wayback Machine, No. 13135, Bostik Corp., 2007-6. Accessed 2012-4-28.
- Samet, Wendy, (comp.), Appendix I, Painting Conservation Catalog, American Institute for Conservation of Historic and Artistic Works, conservation-wiki.com, 1997-9. Accessed 2012-4-28.
- "Cooling baths". UC Davis Chem Wiki. 2013-10-02. Retrieved 8 February 2015.
- Cargille, John (1985) [1964], "Immersion Oil and the Microscope", New York Microscopical Society Yearbook, archived from the original on 2011-09-11, retrieved 2011-03-10
- Carson, Freida; Hladik, Christa (2009). Histotechnology: A Self-Instructional Text (3 ed.). American Society for Clinical Pathology Press. p. 35. ISBN 9780891895817.
- Kandyala, Reena; Raghavendra, Sumanth Phani C.; Rajasekharan, Saraswathi T. (2010-01-01). "Xylene: An overview of its health hazards and preventive measures". Journal of Oral and Maxillofacial Pathology. 14 (1): 1–5. doi:10.4103/0973-029X.64299. ISSN 0973-029X. PMC 2996004. PMID 21180450.
- "ACUTE TOXICITY SUMMARY: XYLENES" (PDF). Archived from the original (PDF) on October 22, 2015.
- "Xylenes (EHC 190, 1997)".
- Gagnaire, F.; Marignac, B.; Langlais, C.; Bonnet, P. (July 2001). "Ototoxicity in rats exposed to ortho-, meta- and para-xylene vapours for 13 weeks". Pharmacology & Toxicology. 89 (1): 6–14. doi:10.1034/j.1600-0773.2001.d01-129.x. ISSN 0901-9928. PMID 11484912.
- Gagnaire, F.; Marignac, B.; Blachère, V.; Grossmann, S.; Langlais, C. (2007-03-07). "The role of toxicokinetics in xylene-induced ototoxicity in the rat and guinea pig". Toxicology. 231 (2–3): 147–158. doi:10.1016/j.tox.2006.11.075. ISSN 0300-483X. PMID 17210216.
- Fuente, Adrian; McPherson, Bradley; Cardemil, Felipe (September 2013). "Xylene-induced auditory dysfunction in humans". Ear and Hearing. 34 (5): 651–660. doi:10.1097/AUD.0b013e31828d27d7. ISSN 1538-4667. PMID 23598724.
- Draper, T. H. J.; Bamiou, D.-E. (April 2009). "Auditory neuropathy in a patient exposed to xylene: case report". The Journal of Laryngology & Otology. 123 (4): 462–465. doi:10.1017/S0022215108002399. ISSN 1748-5460. PMID 18439334.
- Fuente, Adrian; McPherson, Bradley; Hood, Linda J. (November 2012). "Hearing loss associated with xylene exposure in a laboratory worker". Journal of the American Academy of Audiology. 23 (10): 824–830. doi:10.3766/jaaa.23.10.7. ISSN 1050-0545. PMID 23169198.
- "HIPPURIC and METHYL HIPPURIC ACIDS in urine" (PDF). NIOSH Manual of Analytical Methods (NMAM) (Fourth ed.).
- Inoue, O.; Seiji, K.; Kawai, T.; Watanabe, T.; Jin, C.; Cai, S. X.; Chen, Z.; Qu, Q. S.; Zhang, T.; Ikeda, M. (1993). "Excretion of methylhippuric acids in urine of workers exposed to a xylene mixture: Comparison among three xylene isomers and toluene". International Archives of Occupational and Environmental Health. 64 (7): 533–539. doi:10.1007/bf00381104. PMID 8482596.
- Kira S. (1977). "Measurement by gas chromatography of urinary hippuric acid and methylhippuric acid as indices of toluene and xylene exposure". Occupational and Environmental Medicine. 34 (305–309): 305–309. doi:10.1136/oem.34.4.305. PMC 1008281. PMID 588486.
External links
| Wikimedia Commons has media related to Xylenes. |
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica (11th ed.). Cambridge University Press.
- NIOSH Pocket Guide to Chemical Hazards (o-Xylene)
- NIOSH Pocket Guide to Chemical Hazards (m-Xylene)
- NIOSH Pocket Guide to Chemical Hazards (p-Xylene)
- Xylene, Hazard Summary (EPA) (Mixed Isomers)
- The Ear Poisons, The Synergist, American Industrial Hygiene Association, November, 2018