Xanthene
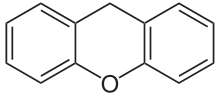
Xanthene (9H-xanthene, 10H-9-oxaanthracene) is the organic compound with the formula CH2[C6H4]2O. It is a yellow solid that is soluble in common organic solvents. Xanthene itself is an obscure compound, but many of its derivatives are useful dyes.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
9H-Xanthene | |
| Other names
Dibenzo[a,e]pyran 10H-9-oxaanthracene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.996 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H10O | |
| Molar mass | 182.222 g·mol−1 |
| Appearance | Yellow solid |
| Melting point | 101 to 102 °C (214 to 216 °F; 374 to 375 K)[1] |
| Boiling point | 310 to 312 °C (590 to 594 °F; 583 to 585 K)[1] |
| Hazards | |
| R-phrases (outdated) | R42 R43 |
| S-phrases (outdated) | S22 S36 S37 S45 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Xanthene dyes
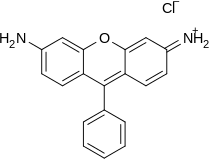
Rhodamines are commercial dyes with xanthene cores.
Dyes that contain a xanthene core include fluorescein, eosins, and rhodamines. Xanthene dyes tend to be fluorescent, yellow to pink to bluish red, brilliant dyes. Many xanthene dyes can be prepared by condensation of derivates of phthalic anhydride with derivates of resorcinol or 3-aminophenol.
Further reading
- Neckers, Douglas C.; Valdes-Aguilera Oscar M. (1993). "Photochemistry of the Xanthene Dyes". Advances in Photochemistry. 18: 315–94. doi:10.1002/9780470133491.ch4.CS1 maint: uses authors parameter (link)
gollark: There are Lisps other than this one, Scheme is just designed for functional programming.
gollark: ... no, it's for homoiconicity.
gollark: "Simple" does not imply "fast to write" (look at Go, although it's more surface-level simple).
gollark: Æ.
gollark: Some of the time it's just stupid, like in `let`s.
See also
References
- Xanthene at Sigma-Aldrich
- Gessner, Thomas; Mayer, Udo (2000). "Triarylmethane and Diarylmethane Dyes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_179.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.