William J. Evans (chemist)
William J. Evans is a distinguished chemistry professor at the University of California Irvine who specialises in the fields of inorganic and organometallic chemistry. He was born in Wisconsin where he also completed his undergraduate studies. He received his bachelor's degree in 1969 after focusing on research into pentaboranes. After the completion of his undergraduate studies he attended The University of California, Los Angeles where he obtained his PhD in 1973 for his research into metallocarboranes.[1] He then spent some time at Cornell University where he completed his postdoctoral research in transition metal phosphite complexes. In 1975, Evans arrived at the University of Chicago where he decided to begin research into actinides and lanthanides1. He moved to the University of California, Irvine in 1983 where he has remained ever since1.Evans has won many accolades which include the ACS Award in Inorganic Chemistry in 2005 and the prestigious Tolman award in 2014 for his research in the field of coordination chemistry of the late elements. [2] Evans has a particular interest in meeting the global energy demand. His research focuses on the role that actinides and lanthanides can play in these processes3. Evans and his research group believe that the unique properties displayed by these rare metals, could help to solve global problems through potential new catalysis and improved processes.[3]
.png)
Past research
Evans discovered a series of sterically crowded Tris(pentamethylcyclopentadienyl) [(C5Me5)3M] complexes from the synthetic study of (C5Me5)2Sm.[4] This discovery was significant because of the metal-ligand bond lengths in these complexes which were 0.1 Å longer than conventional distances.[4] With lanthanide and actinides metal ions (La, Ce, Pr, Nd, Sm, Gd, Y, and U), the complex can also react with nitriles [Me3CNN] and isocyanide [Me3CNC].[5][6]
Over the past several decades, Evans has shown that lanthanides despite previously being deemed as generally redox inactive metal sites, can undergo well-defined one-electron redox processed. By using sterically-encumbering cyclopentadienyl-type ligands, his group was able to perform reduction chemistry on a number of organolanthanide species thereby isolating these metal complex in new oxidation states. These include the isolation of: [(C5Me5)3La(NCCMe3)2],[7] [(C5Me5)3Ce(NCCMe3)2], [(C5Me5)3Nd(NCCMe3)], [(C5Me5)3U(NCCMe3)], and [(C5Me5)2Sm{NC(C5Me5)(CMe3)}(NCCMe3)].[6]
Recent research
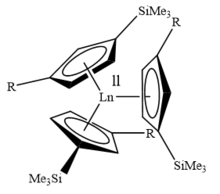
In recent years William Evans has focused on discovering new oxidation states for different heavy metals. In 2017 he discovered a new oxidation state of Scandium. He synthesised certain complexes that contain Sc2+ as opposed to the usual +3 oxidation state.[8] In the same year, Evans also discovered a new oxidation state of Pu; the +2 oxidation state, namely Pu2+ in [K(2.2.2-cryptand)][Pu(II)Cp″3], Cp″ = C5H3(SiMe3)2.[9] This was in contrast to the more familiar +3, +4, +5, +6, and +7 oxidation states. Most recently, Evans analyzed the synthesis of the oxidation-reduction reaction of complex (C5Me5)2ThMe2, where thorium was reduced from the +4 oxidation state to the +3 oxidation state.[10]
References
- http://scalacs.org/?page_id=2467 Southern California Section of the American Chemical Society, 2014 William J. Evans, UC Irvine, 8 May 2017
- University of California Irvine, William J. Evans, 8 May 2017
- University of California Irvine, Evans Group Lanthanide and Actinide Chemistry, 8 May 2017
- Evans, William J. (2007). "The Importance of Questioning Scientific Assumptions: Some Lessons from f Element Chemistry†". Inorganic Chemistry. 46 (9): 3435–3449. doi:10.1021/ic062011k. PMID 17428046.
- Evans, William J.; Perotti, Jeremy M.; Kozimor, Stosh A.; Champagne, Timothy M.; Davis, Benjamin L.; Nyce, Gregory W.; Fujimoto, Cy H.; Clark, Robert D.; Johnston, Matthew A. (2005-08-01). "Synthesis and Comparative η1-Alkyl and Sterically Induced Reduction Reactivity of (C5Me5)3Ln Complexes of La, Ce, Pr, Nd, and Sm". Organometallics. 24 (16): 3916–3931. doi:10.1021/om050402l. ISSN 0276-7333.
- William J. Evans, Thomas J. Mueller, Joseph W. Ziller (2010). "Lanthanide versus Actinide Reactivity in the Formation of Sterically Crowded (C5Me5)3MLn Nitrile and Isocyanide complexes" (PDF). Chemistry: A European Journal. 16 (3): 964–975. doi:10.1002/chem.200901990. PMID 19946904.CS1 maint: multiple names: authors list (link)
- Evans, William J.; Mueller, Thomas J.; Ziller, Joseph W. (2009-02-25). "Reactivity of (C5Me5)3LaLx Complexes: Synthesis of a Tris(pentamethylcyclopentadienyl) Complex with Two Additional Ligands, (C5Me5)3La(NCCMe3)2". Journal of the American Chemical Society. 131 (7): 2678–2686. doi:10.1021/ja808763y. ISSN 0002-7863. PMID 19166353.
- Woen, DH; Chen, GP; Ziller, JW; Boyle, TJ; Furche, F; Evans, WJ (2017). "Solution Synthesis, Structure, and CO2 Reduction Reactivity of a Scandium(II) Complex, {Sc[N(SiMe3)2]3}−". Angew Chem Int Ed Engl. 56 (8): 2050–2053. doi:10.1002/anie.201611758. PMID 28097771.
- Windorff, Cory J. (2017). "Identification of the Formal +2 Oxidation State of Plutonium: Synthesis and Characterization of {PuII[C5H3(SiMe3)2]3}". Journal of the American Chemical Society. 139 (11): 3970–3973. doi:10.1021/jacs.7b00706. PMID 28235179.
- Langeslay, Ryan R.; Chen, Guo P.; Windorff, Cory J.; Chan, Alan K.; Ziller, Joseph W.; Furche, Filipp; Evans, William J. (2017). "Synthesis, Structure, and Reactivity of the Sterically Crowded Th3+ Complex (C5Me5)3Th Including Formation of the Thorium Carbonyl, [(C5Me5)3Th(CO)][BPh4]". Journal of the American Chemical Society. 139 (9): 3387–3398. doi:10.1021/jacs.6b10826. PMID 28240899.