William B. Tolman
William B. Tolman is an inorganic chemist whose laboratory works on synthesis and characterization of bioinorganic systems, organometallic reagents and polymers. He received his bachelor's degree in Chemistry from Wesleyan University in 1983, and then completed a Ph.D. program in Chemistry at the University of California, Berkeley by 1987 under the guidance of Professor K. Peter C. Vollhardt.[1][2] He previously was employed at the University of Minnesota as the Department Chair of Chemistry. Currently, he is the William Greenleaf Eliot Professor of Chemistry at Washington University in St. Louis. In addition to his faculty position, he is also the Editor and Chief of the ACS journal of Inorganic Chemistry.[3]
Copper-Oxygen Adducts
Tolman's work in the bioinorganic field focuses on Cu-O adducts, specifically copper proteins whose diverse, biological functions include: O2 transport, aromatic ring oxidations, biogenesis of hormones.[4] His work studies the potential of 1:1 Cu/O2 adducts as catalytic species, which have been known as transient intermediates for more commonly studied 2:1 and even 3:1 Cu/O2 molecules. These complexes, while kinetically favored in formation are thermodynamically unstable due to negative entropy values, thus making them more difficult to isolate.[4] Although, increasing ligand sizes on these 1:1 adducts did correlate with slower reaction rate constants; advantageous for isolating and studying these complexes.[3]
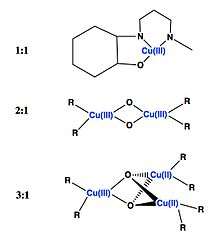
Furthermore, his work on high and mixed valent copper species including [CuOH]+2 and its conjugate base, [CuO]+ is also very notable. His work with [CuOH]+2 reveals a high reactivity with C-H and O-H bonds as compared to its conjugate acid pair.[3] This is of importance when trying to replicate biological mechanisms, such as copper-catalyzed oxidation in vitro.
His research has greatly contributed to the discovery and characterization of new biomimetic species. It is his goal to not only identify these compounds, but to comprehensively understand the intermediates and mechanisms with which they play crucial roles in facilitating. In the case of Cu/O2 adducts, realizing their biological role and function in copper containing enzymes can give rise to new insights on their biomimetic properties.[3]
Additionally, his lab is searching for alternative, synthetic oxidative catalysis. This includes designing biochemically-inspired synthetic catalysts as well as trying O2 as a candidate for controlled, in vitro oxidation. Due to high abundances and relatively strong stabilizing capabilities within biological reactions, iron and copper enzymes inspire biomimetic synthetic catalysts. Although these reactions perform with high accuracy and selectivity within the body, many challenges arise when working with O2 in vitro because of the undesired and potentially harmful side products that can be generated.[5]
Organometallic Catalysts
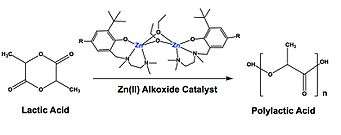
An example of his organometallic work includes Zn(II) and Fe(III) alkoxide catalysts, used to polymerize lactide (LA) into polylactide (PLA).[6][7] [4] PLA is of great interest because it is both biodegradable and a renewable resource.[6][8] While there are many well known catalysts available to synthesize PLA, not much is known about these mechanisms; proving problematic in designing new and efficient catalysts. In an attempt to resolve this, less structurally complex catalysts are being produced and characterized.[9] Tolman's research in accordance, provided that catalysts with lower coordination numbers have higher polymerization activities.[7] His Zn(II) alkoxide catalyst, as an example, produced PLA with a high molecular weight at a relatively fast rate.
References
- Tolman, William B. (1997). "Making and Breaking the Dioxygen O−O Bond: New Insights from Studies of Synthetic Copper Complexes". Accounts of Chemical Research. 30 (6): 227–237. doi:10.1021/ar960052m.
- "Tolman Group Laboratory". www1.chem.umn.edu. Retrieved 2017-06-06.
- Elwell, Courtney E.; Gagnon, Nicole L.; Neisen, Benjamin D.; Dhar, Debanjan; Spaeth, Andrew D.; Yee, Gereon M.; Tolman, William B. (2017-02-08). "Copper–Oxygen Complexes Revisited: Structures, Spectroscopy, and Reactivity". Chemical Reviews. 117 (3): 2059–2107. doi:10.1021/acs.chemrev.6b00636. ISSN 0009-2665. PMC 5963733. PMID 28103018.
- Lewis, Elizabeth A.; Tolman, William B. (2004). "Reactivity of Dioxygen−Copper Systems". Chemical Reviews. 104 (2): 1047–1076. doi:10.1021/cr020633r. PMID 14871149.
- Que, Lawrence; Tolman, William B. (2008-09-18). "Biologically inspired oxidation catalysis". Nature. 455 (7211): 333–340. doi:10.1038/nature07371. ISSN 0028-0836. PMID 18800132.
- O'Keefe, Brendan J.; Breyfogle, Laurie E.; Hillmyer, Marc A.; Tolman, William B. (2002). "Mechanistic Comparison of Cyclic Ester Polymerizations by Novel Iron(III)−Alkoxide Complexes: Single vs Multiple Site Catalysis". Journal of the American Chemical Society. 124 (16): 4384–4393. doi:10.1021/ja012689t. PMID 11960467.
- Williams, Charlotte K.; Breyfogle, Laurie E.; Choi, Sun Kyung; Nam, Wonwoo; Young, Victor G.; Hillmyer, Marc A.; Tolman, William B. (2003). "A Highly Active Zinc Catalyst for the Controlled Polymerization of Lactide". Journal of the American Chemical Society. 125 (37): 11350–11359. doi:10.1021/ja0359512. PMID 16220958.
- Kuehner, Marcel. "Bioplastics - Study: Market, Analysis, Trends | Ceresana". www.ceresana.com. Retrieved 2017-06-06.
- O'Keefe, Brendan J.; Hillmyer, Marc A.; Tolman, William B. (2001-01-01). "Polymerization of lactide and related cyclic esters by discrete metal complexes". Journal of the Chemical Society, Dalton Transactions. 0 (15): 2215–2224. doi:10.1039/B104197P. ISSN 1364-5447.