Wesley Sundquist
Wesley Sundquist (born 1959) is an American chemists and biochemist. Sundquist is known for studying the cellular, molecular and structurally biology of retroviruses, particularly HIV. He is also known for studying the ESCRT pathway in cell division.[1]
Life and education

Wesley Sundquist was born in St. Paul, Minnesota in 1959. He grew up in St. Paul Minnesota and Washington, DC. He received his bachelor’s degree in Chemistry from Carleton College in Minnesota in 1981. During his time at Carleton Sundquist served as the coordinator of the Faribault Project, was elected to Sigma Xi and received a National Merit Scholarship (1977-81). Sundquist went on to complete a PhD at the Massachusetts Institute of Technology in 1988. Following his PhD, he went on to participate in postdoctoral research at the MRC Laboratory of Molecular in Cambridge, England under Sir Aaron Klug. In 1992 Sundquist joined the University of Utah biochemistry department.[2] Sundquist is married to Nola Sundquist, with whom he lives with in Salt Lake City, Utah. They have two adult children, Chris and Emily.
Work and discoveries
Sundquist is Distinguished Professor and Chair of the Department of Biochemistry at the University of Utah, and a member of the Cell Response and Regulation Program at the Huntsman Cancer Institute.[1] He also directs a research lab. The Sundquist Lab focuses on cellular, molecular and structural biology of retroviruses with a focus on Human Immunodeficiency Virus, HIV. Major projects in the lab include, 1) enveloped virus assembly 2) ESCRT pathway functions and regulation in cell division and cancer, and 3) HIV capsid structure, replication and restriction.[3]
Enveloped virus assembly
In order for HIV to leave a cell and spread infection, it must be enveloped within a. membrane. Retroviruses like HIV bud from infected cells using the Endosomal Sorting Pathway Required for Transport or ESCRT pathway. In addition to this pathway, HIV also uses the host proteins of the Angiomotin family in facilitating membrane envelopment prior to ESCRT- mediated budding. Sundquist's primary research in this area focuses on understanding assembly and budding of HIV, characterizing the host and viral proteins involved, and testing restriction of viruses using the ESCRT due to our innate immune proteins. The Sundquist lab has also used their understanding of the requirements and principles of enveloped virus assembly to design and characterize new proteins that can assemble into nanocages, bud from cells, and carry cargoes into new target cells.
Selected publications
- Garrus JE, von Schwedler UK, Pornillos O, Morham SG, Zavitz KH, Wang HE, Wettstein DA, Stray KM, Cöté M, Rich RL, Myszka DG, Sundquist WI. (2001). Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell, 107, 55-65. PMID: 11595185
- von Schwedler U, Stuchell M, Müller B, Ward D, Chung H-Y, Morita E, Wang H, Davis T, Gong-Ping H, Cimbora DM, Scott AT, Kräusslich H-G, Kaplan J, Morham SG, and Sundquist WI. (2003). The protein network of HIV budding. Cell, 114, 701-713. PMID: 14505570
- Mercenne G, Alam SL, Arii J, Lalonde MS, Sundquist WI. (2015). Angiomotin functions in HIV-1 assembly and budding. eLife, 4, e03778. PMCID: PMC4337731
- Votteler J, Ogohara C, Yi S, Hsia Y, Natterman U, Belnap DM, King NP, Sundquist WI. (2016). Designed proteins induce the formation of nanocage-containing extracellular vesicles. Nature, 540, 292-5. PMID: 27919066
ESCRT pathway functions and cell division
Multi-vesicular body biogenesis, neuronal pruning, reassembly of the post-mitotic nuclear envelope and final stage cell division are all catalyzed through the ESCRT pathway. Cytokinetic abscission completes the separation of the two daughter cells, and also helps to coordinate the checkpoint that delays cell division until mitotic processes are complete. The ESCRT pathway mediates the recruiting and organizing of this final mechanical step of abscission. Within some cancer cells, the function of this pathway doesn’t function correctly. In studying this process, Sundquist’s lab is determining the structures and functions associated with the filaments formed by the ESCRT-III proteins and the proteins they recruit to help mediate abscission and the abscission checkpoint. Signaling pathways that control the checkpoint and ESCRT pathway are also explored.
Selected publications
- Scott, A.; Gaspar, J.; Stuchell-Brereton, M. D.; Alam, S. L.; Skalicky, J. J.; Sundquist, W. I. (2005). "Structure and ESCRT-III protein interactions of the MIT domain of human VPS4A". Proceedings of the National Academy of Sciences. 102 (39): 13813–13818. Bibcode:2005PNAS..10213813S. doi:10.1073/pnas.0502165102. PMC 1236530. PMID 16174732.
- Langelier, Charles; von Schwedler, Uta K.; Fisher, Robert D.; De Domenico, Ivana; White, Paul L.; Hill, Christopher P.; Kaplan, Jerry; Ward, Diane; Sundquist, Wesley I. (2006). "Human ESCRT-II Complex and Its Role in Human Immunodeficiency Virus Type 1 Release". Journal of Virology. 80 (19): 9465–9480. doi:10.1128/JVI.01049-06. PMC 1617254. PMID 16973552.
- Kieffer, Collin; Skalicky, Jack J.; Morita, Eiji; De Domenico, Ivana; Ward, Diane M.; Kaplan, Jerry; Sundquist, Wesley I. (2008). "Two Distinct Modes of ESCRT-III Recognition Are Required for VPS4 Functions in Lysosomal Protein Targeting and HIV-1 Budding". Developmental Cell. 15 (1): 62–73. doi:10.1016/j.devcel.2008.05.014. PMC 2586299. PMID 18606141.
- McCullough, J.; Clippinger, A. K.; Talledge, N.; Skowyra, M. L.; Saunders, M. G.; Naismith, T. V.; Colf, L. A.; Afonine, P.; Arthur, C.; Sundquist, W. I.; Hanson, P. I.; Frost, A. (2015). "Structure and membrane remodeling activity of ESCRT-III helical polymers". Science. 350 (6267): 1548–1551. Bibcode:2015Sci...350.1548M. doi:10.1126/science.aad8305. PMC 4684769. PMID 26634441.
- Stuchell-Brereton MD, Skalicky JJ, Kieffer C, Karren MA, Ghaffarian S, Sundquist WI. ESCRT-III recognition by VPS4 ATPases. (2007) Nature, 449, 740-744. PMID: 17928862
- Morita E, Sandrin V, Chung HY, Morham SG, Gygi SP, Rodesch CK, Sundquist WI. (2007). Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J, 26(19), 4215-27. PMCID: PMC2230844
- Caballe A, Wenzel DM, Agromayor M, Alam SL, Skalicky JJ, Kloc M, Carlton JG, Labrador L, Sundquist WI, Martin-Serrano J (2015). ULK3 regulates cytokinetic abscission by phosphorylating ESCRT-III proteins. eLife, May 26;4. PMCID: PMC4475061
HIV replication and restriction
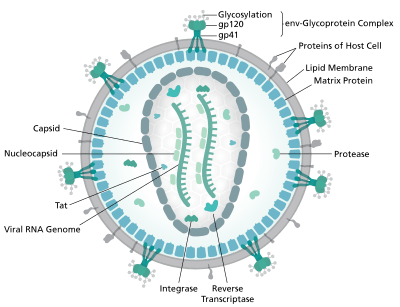
The capsid of HIV facilitates viral reverse transcription and protects the viral genome from the innate immune system. Sundquist defined the fullerene cone structure of the viral capsid and has helped to define how the host restriction factor, TRIM5a recognizes and assembles around the capsid. Furthering these findings, the Sundquist Lab is investigating exactly how the capsid promotes reverse transcription and other stages of viral replication.
Selected publications
- Ganser BK, Li S, Klishko VY, Finch JT, Sundquist WI. (1999). Assembly and analysis of conical models for the HIV-1 core. Science, 283(5398), 80-3. PMID: 9872746
- Li S, Hill CP, Sundquist WI, Finch JT. (2000). Image reconstructions of helical assemblies of the HIV-1 CA protein. Nature, 407(6802), 409-13. PMID: 11014200
- Pornillos O, Ganser-Pornillos BK, Kelly BN, Hua Y, Whitby FG, Stout CD, Sundquist WI, Hill CP, Yeager M. (2009). X-ray structures of the hexameric building block of the HIV capsid. Cell, 137(7), 1282-92. PMCID: PMC2840706.
- Li, Yen-Li; Chandrasekaran, Viswanathan; Carter, Stephen D.; Woodward, Cora L.; Christensen, Devin E.; Dryden, Kelly A.; Pornillos, Owen; Yeager, Mark; Ganser-Pornillos, Barbie K.; Jensen, Grant J.; Sundquist, Wesley I. (2016). "Primate TRIM5 proteins form hexagonal nets on HIV-1 capsids". eLife. 5. doi:10.7554/eLife.16269. PMC 4936896. PMID 27253068.
- Wagner, Jonathan M.; Christensen, Devin E.; Bhattacharya, Akash; Dawidziak, Daria M.; Roganowicz, Marcin D.; Wan, Yueping; Pumroy, Ruth A.; Demeler, Borries; Ivanov, Dmitri N.; Ganser-Pornillos, Barbie K.; Sundquist, Wesley I.; Pornillos, Owen (2017). "A general model for retroviral capsid pattern recognition by TRIM5 proteins". Journal of Virology. 92 (4): JVI.01563–17. doi:10.1128/JVI.01563-17. PMC 5790955. PMID 29187540.
- Sundquist, Wesley I.; Pornillos, Owen (2018). "Retrovirus Restriction by TRIM5α: RINGside at a Cage Fight". Cell Host & Microbe. 24 (6): 751–753. doi:10.1016/j.chom.2018.11.013. PMC 6464375. PMID 30543772.
Honors and scientific legacy
In 1993 Sundquist received the Searle Scholars Award.
In 2003 he received the ASBMB Amgen Award for the Application of Biochemistry and Molecular Biology to the understanding of disease.
In 2004 he received both the MERIT award from the National Institutes of Health and the Bernard Fields award for Retrovirology.
In 2017 he received the University of Utah Rosenblatt Prize for Excellence.
He has been elected to the American Academy of Arts and Sciences (2011) and the National Academy of Sciences.[2] (2014).
References
- "Members - U of U School of Medicine - | University of Utah". medicine.utah.edu. Retrieved 2020-04-08.
- "Wesley I. Sundquist '81 | Class of 1981 | Carleton College". apps.carleton.edu. Retrieved 2020-04-08.
- "Research - | University of Utah". medicine.utah.edu. Archived from the original on 2020-02-11. Retrieved 2020-04-08.