Weak-Link Approach
The Weak-Link Approach (WLA) is a supramolecular coordination-based assembly methodology, first introduced in 1998 by the Mirkin Group at Northwestern University.[1] This method takes advantage of hemilabile ligands, containing both strong and weak binding moieties, that can coordinate to metal centers and quantitatively assemble into a single condensed ‘closed’ structure (Figure 1). Unlike other supramolecular assembly methods, the WLA allows for the synthesis of supramolecular complexes that can be modulated from rigid ‘closed’ structures to flexible ‘open’ structures through reversible binding events that occur at the structural metal centers. The approach is general and has been applied to a variety of metal centers and ligand designs (Figure 2) including those with utility in catalysis and allosteric regulation.
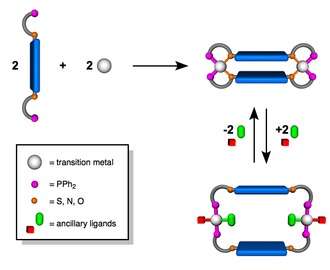
Classes of Allosteric Structures Assembled via the WLA
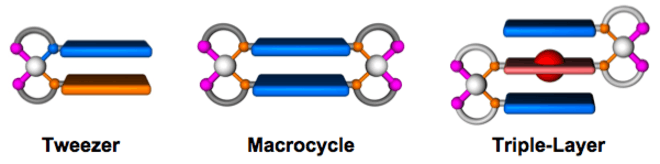
Metal Precursors Utilized in the WLA
To date, Rh(I), Ir(I), Pd(II), Ru(II), Cu(I), Ni(II), and Pt(II) have been used as metal precursors in the WLA.
Hemilabile Ligands Utilized in the WLA
The key component that enables in situ control of supramolecular architecture via the WLA is the use of hemilabile ligands.[2] Hemilabile ligands are polydentate chelates that contain at least two different types of bonding groups, denoted X and Y (Figure 3). The first group (X) bonds strongly to the metal center, while the other group (Y) is weakly bonding and easily displaced by coordinating ligands or solvent molecules (Z). In this way, the substitutionally labile group (Y) can be displaced from, yet remain available for recoordination to, the metal center. For WLA-generated structures, a typical ligand design consists of a phosphine-based strong binding group and a weak-binding group containing O, S, Se, or N.
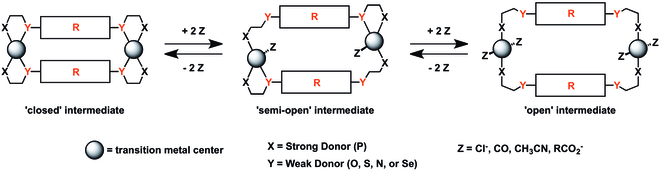
The use of hemilabile ligands allows structural motifs synthesized via the WLA to be modified in situ with small molecule effectors much like allosteric enzymes in biology. As described above, the weak Y–M bond can be easily displaced by a variety of other coordinating ligands including: Cl−, CO, CH3CN, RCO2−, and a variety of nitriles/isonitriles. Utilizing different amounts of ancillary ligand, the researcher can toggle between a number of complex conformations. In the closed state, the metal center is fully chelated to both ligands. With the addition of one equivalent of the selected ancillary ligand, a semi-open complex is formed which involves one ligand fully chelated and the other bound to the metal only through the phosphorus moiety. In mixed ligand systems, the ancillary ligand will selectively displace the weakest Y–M bond - an important feature for the more sophisticated catalytic structures (based on the halide-induced ligand rearrangement (HILR) reaction[3][4] and triple-layer geometries). Lastly, with the introduction of two equivalents of ancillary ligand, the fully open complex is formed where both ligands are bound only through the phosphorus moiety. Importantly, this process is completely reversible in all cases with the addition of appropriate abstraction agents or in some cases by applying vacuum to the system.
Examples of Functional Allosteric Structures
Allosteric regulation in supramolecular structures generated via the WLA is particularly important in the context of designing and synthesizing novel, bioinspired catalytic systems, where the conformation of the complex controls the activity of the catalyst. Below are a series of different catalytic motifs that have been constructed via the WLA and a discussion of the control mechanisms that can be used to modulate catalytic activity:
ELISA Mimic[5]
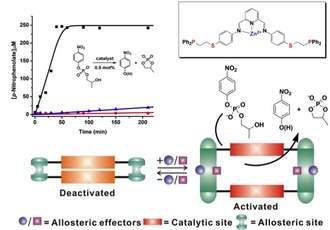
The first catalytically active supramolecular structure generated via the WLA was designed to operate via a mechanism inspired by the Enzyme Linked ImmunoSorbent Assay (ELISA). In such a supramolecular system, a target sandwiching event creates a catalyst target complex that subsequently generates a chemiluminescent or fluorescent readout. For example, a homoligated WLA-based Rh(I) macrocyclic structure has been developed that incorporates pyridine-bisimine Zn(II) moieties and behaves as an efficient and completely reversible allosteric modulator for the hydrolysis of 2-(hydroxypropyl)-p-nitrophenyl phosphate (HPNP), a model substrate for RNA. Significantly, the structural changes induced by small molecule regulators Cl− and CO transition this system from a catalytically inactive state to a very active one in a highly reversible fashion.
Further, this system provides a highly sensitive platform for sensing chloride anions. As chloride binds to the Rh(I) centers, the complex is opened allowing hydrolysis to occur. The hydrolysis product of the reaction (p-nitrophenolate) can be followed by UV-Vis spectroscopy. As in ELISA, the WLA-generated mimic is able to take a small amount of target (chloride anions) and produce a large fluorescent readout that can be utilized for detection.
There are several notable conclusions that can be drawn based on the catalytic studies of this complex. The first is that the closed complex is completely inactive under hydrolysis conditions. Second, the open complex is extremely active and capable of quantitatively hydrolyzing all of the HPNP substrate in less than 40 min. By simply bubbling N2 into the solution, the reformation of the closed complex and the generation of an inactive catalyst can be achieved.
PCR Mimic[6]
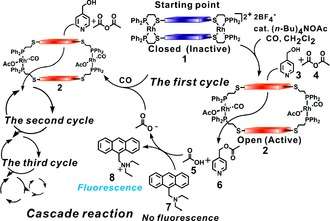
The polymerase chain reaction (PCR) is utilized in biochemistry and molecular biology for exponentially amplifying nucleic acids by making copies of a specific region of a nucleic acid target. When coupled with diagnostic probes, this technique allows one to detect a small collection of molecules under very dilute conditions. A limitation of PCR is that it only works with nucleic acid targets, and there are no known analogues of PCR for other target molecular candidates.
Using the WLA, this type of target amplification approach has been exemplified in an abiotic system. By incorporating Zn(II)-salen ligands into a supramolecular assembly, an acyl transfer reaction involving acetic anhydride and pyridylcarbinol as substrates was investigated. In the absence of acetate, there is almost no catalytic activity. Once a small amount of tetrabutylammonium acetate reacts with inactive complex at its two rhodium centers that serve as structural regulatory sites, the complex is converted into open cavity complex, which then catalyzes the reaction.
In the early stages of the reaction, only a minor amount of the catalyst is activated. As the reaction proceeds, more acetate is generated, which leads to the formation of more activated complex and progressively faster catalysis. This type of behavior is typical for cascade-type reactions including PCR. Unlike the previous example in which the catalyst produced a signal amplifier, this catalyst is a target amplifier making more copies of the target acetate. Following the reaction by gas chromatography, one observes that the generation of products follows a sigmoidal curve, indicative of a PCR-like cascade reaction system.
Triple-Layer Structure[7]
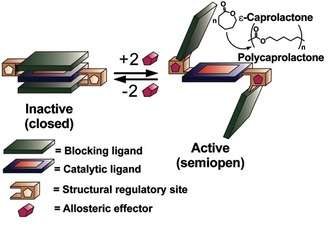
Extending the idea of heteroligated structures, there was also a need to design a catalytic structure that would allow for the inclusion of mono-metallic catalyst that could be completely turned off. To this end the triple-layer motif was developed, composed of two transition metal nodes, two chemically inert blocking exterior layers, and a single catalytically active interior ligand. This complex was synthesized using the WLA and HILR processes, and it can be reversibly activated and deactivated through small-molecule or elemental anion effector reactions that assemble and disassemble the trilayer structures. In the recent Al(III)-salen example, the polymerization of ε-caprolactone could be turned on and off based on the ancillary ligands and abstraction agents added to the system. Unlike in previous catalytic structures that utilized bimetallic systems, utilizing the tri-layer motif allows for the incorporation of a monometallic catalyst, opening the scope of potential catalysts that can be employed in these types of structures.
References
- Farrell, Joshua R.; Mirkin, Chad A.; Guzei, Ilia A.; Liable-Sands, Louise M.; Rheingold, Arnold L. (1998). "The Weak-Link Approach to the Synthesis of Inorganic Macrocycles". Angewandte Chemie International Edition. 37 (4): 465. doi:10.1002/(SICI)1521-3773(19980302)37:4<465::AID-ANIE465>3.0.CO;2-A.
- Jeffrey, J. C.; Rauchfuss, T. B. (1979). "Metal complexes of hemilabile ligands. Reactivity and structure of dichlorobis(o-(diphenylphosphino)anisole)ruthenium(II)". Inorganic Chemistry. 18 (10): 2658. doi:10.1021/ic50200a004.
- Brown, A. M.; Ovchinnikov, M. V.; Stern, C. L.; Mirkin, C. A. (2004). "Halide-Induced Supramolecular Ligand Rearrangement". Journal of the American Chemical Society. 126 (44): 14316–14317. doi:10.1021/ja045316b. PMID 15521726.
- Oliveri, C. G.; Ulmann, P. A.; Wiester, M. J.; Mirkin, C. A. (2008). "Heteroligated Supramolecular Coordination Complexes Formed via the Halide-Induced Ligand Rearrangement Reaction". Accounts of Chemical Research. 41 (12): 1618–1629. doi:10.1021/ar800025w. PMID 18642933.
- Gianneschi, N. C.; Bertin, P. A.; Nguyen, S. T.; Mirkin, C. A.; Zakharov, L. N.; Rheingold, A. L. (2003). "A Supramolecular Approach to an Allosteric Catalyst". Journal of the American Chemical Society. 125 (35): 10508–10509. doi:10.1021/ja035621h. PMID 12940719.
- Yoon, H. J.; Mirkin, C. A. (2008). "PCR-like Cascade Reactions in the Context of an Allosteric Enzyme Mimic". Journal of the American Chemical Society. 130 (35): 11590–11591. doi:10.1021/ja804076q. PMID 18681433.
- Yoon, H. J.; Kuwabara, J.; Kim, J. -H.; Mirkin, C. A. (2010). "Allosteric Supramolecular Triple-Layer Catalysts". Science. 330 (6000): 66–69. Bibcode:2010Sci...330...66Y. doi:10.1126/science.1193928. PMID 20929805.