Vosaroxin
Vosaroxin (AG-7352, SPC-595, SNS 595, voreloxin) is a topoisomerase II inhibitor causing site-selective DNA damage. It is under phase III clinical trial investigation for acute myelogenous leukemia (AML) and ovarian cancer sponsored by Sunesis.[1]
 | |
| Names | |
|---|---|
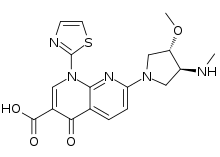
| IUPAC name
7-[(3S,4S)-3-Methoxy-4-(methylamino)-1-pyrrolidinyl]-4-oxo-1-(1,3-thiazol-2-yl)-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid | |
| Other names
Voreloxin | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H19N5O4S | |
| Molar mass | 401.44 g·mol−1 |
| Density | 1.5±0.1 g/cm3 |
| Pharmacology | |
| L01XX53 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mechanism of action
Vosaroxin is a naphthyridine analog of the anticancer quinolone derivatives (AQDs), a class of compounds that has not been used previously for the treatment of cancer. Topoisomerase II enzymes are essential for the survival of eukaryotic cells. Vosaroxin hinders the reunion of topoisomerase II-induced double-strand breaks at selective sites in DNA, resulting in G2 arrest and cell death by apoptosis.[2]
gollark: Anyway, Revolution IRC is probably on F-droid thus APIONET (join).
gollark: To be fair it does that often.
gollark: irc.osmarks.tk or irc.tty0.xyz.
gollark: If Discord breaks more just switch to APIONET.
gollark: Is osmarks.tk up?
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.