von Braun reaction
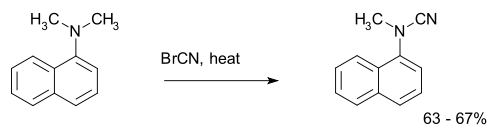
The von Braun reaction is an organic reaction in which a tertiary amine reacts with cyanogen bromide to an organocyanamide.[1] An example is the reaction of dimethyl-α-naphthylamine:[2]
| von Braun reaction | |||||||
|---|---|---|---|---|---|---|---|
| Named after | Julius von Braun | ||||||
| Reaction type | Substitution reaction | ||||||
| Reaction | |||||||
| |||||||
Reaction mechanism
The reaction mechanism consists of two nucleophilic substitutions: the amine is the first nucleophile displacing the bromine atom which then acts as the second nucleophile.[3][4] In following the mechanism is described using trimethylamine as example:[5]
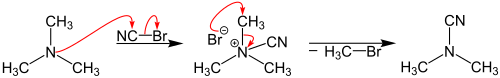
First, the trimethylamine reacts with the cyanogen bromide to form a cyano group. This results in a quaternary ammonium salt, which in the next step reacts by splitting off bromomethane to give the dimethylcyanamide. This is a second-order nucleophilic substitution (SN2).
gollark: Sure? But never underestimate the patience of people doing stupid things.
gollark: Ah yes.
gollark: But it is said that locks only work for keeping out honest people, inasmuch as they can be bypassed or picked or whatever quite easily.
gollark: What are you responding to here?
gollark: Also, that's price discrimination and very dodecahedral.
See also
References
- J. von Braun; K. Heider & E. Müller (1918). "Bromalkylierte aromatische Amine. II. Mitteilung". Chem. Ber. 51 (1): 273–282. doi:10.1002/cber.19180510132.
- Homer W. J. Cressman (1947). "N-Methyl-1-Naphthylcyanamide". Org. Synth. 27: 56. doi:10.15227/orgsyn.027.0056.
- March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- Howard A. Hageman (1953). "The Von Braun Cyanogen Bromide Reaction". Organic Reactions. 7 (4): 198–262. doi:10.1002/0471264180.or007.04. ISBN 0471264180.
- Jie Jack Li (2014), Name reactions: A collection of detailed mechanisms and synthetic applications (in German) (5th ed.), Cham: Springer, p. 619, doi:10.1007/978-3-319-03979-4, ISBN 978-3-319-03979-4
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.