Violanthrone
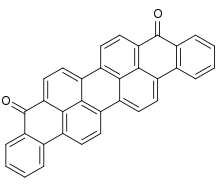
Violanthrone, also known as Dibenzanthrone, is an organic compound that serves as a vat dye and a precursor to other vat dyes. X-ray crystallography confirms that the molecule is planar with C2v symmetry.[1] Isomeric with violanthrone is isoviolanthrone, which has a centrosymmetric structure.[2]
 | |
| Names | |
|---|---|
| Other names
Dibenzanthrone, Tinon Dark Blue BOA, Ahcovat Dark Blue BO, Violanthrone A, Bianthrone A, Irgalite Blue 2R, Paradone Dark Blue | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.775 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C34H16O2 | |
| Molar mass | 456.48964 |
| Appearance | dark blue solid |
| Density | 1.53 g/cm3 |
| -204.8·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
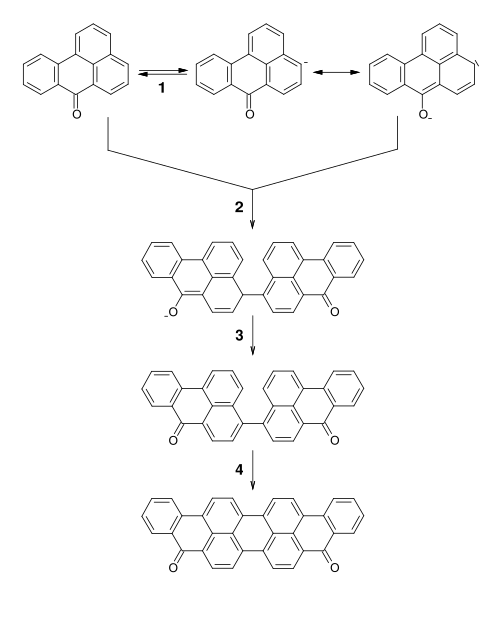
Synthesis
It is produced by coupling of two molecules of benzanthrone.[3][4]
gollark: You could contribute code to osmarks.tk.
gollark: Which is odd, because it appears to be a water pistol.
gollark: Please explain.
gollark: What is maths doing here?
gollark: How is that related to π?!
References
- The crystal structure of violanthrone (dibenzanthrone) Bolton, W.; Stadler, H. P. Acta Crystallographica 1964, volume 17, pp. 1015-20. doi: 10.1107/S0365110X64002584
- Bien, H.-S.; Stawitz, J.; Wunderlich, K. "Anthraquinone Dyes and Intermediates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_355.
- Manufacture of dibenzanthrone compounds
- Heinrich Zollinger, Color Chemistry: Syntheses, Properties, and Applications of Organic Dyes and Pigments, 3rd edition, WILEY-VCH, Weinheim, 2003, ISBN 3-906390-23-3, p. 291
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.