Vinyllithium
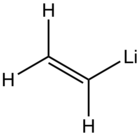
Vinyllithium is an organolithium compound with the formula LiC2H3. A colorless or white solid, it is encountered mainly as a solution in tetrahydrofuran (THF). It is a reagent in synthesis of organic compounds.[1]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| |
| |
| Properties | |
| C2H3Li | |
| Molar mass | 33.99 g·mol−1 |
| Appearance | white solid |
| Hazards | |
| Main hazards | pyrophoric |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and structure
Solutions of vinyllithium are prepared by lithium-halogen exchange reactions. A halide-free route entails reaction of tetravinyltin with butyllithium:
- Sn(CH=CH2)4 + 4 BuLi → SnBu4 + 4 LiCH=CH2
The reaction of ethylene and lithium affords vinyl lithium and lithium hydride, together with other organolithium compounds,[1]
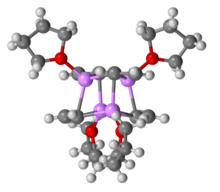
Like most organolithium compounds, vinyllithium crystallizes from THF as a cluster compound as a cubane-type cluster.[2]
Reactions
Vinyllithium is used to install vinyl groups on metal-based reagents, being a precursor to vinylsilanes, vinylcuprates, and vinylstannanes.[3] It adds to ketones compounds to give allylic alcohols. Vinylmagnesium bromide is often used in place of vinyllithium.[4]
References
- Eisenhart, Eric K.; Bessieres, Bernard (2007). "Vinyllithium". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rv015.pub2.CS1 maint: uses authors parameter (link).
- Walter Bauer, Frank Hampel (1992). "X-Ray crystal structure of a vinyllithium–tetrahydrofuran solvate (C2H3Li–thf)4. Quantitative estimation of Li–H distances by 6Li–1H HOESY". J. Chem. Soc., Chem. Commun.: 903–905. doi:10.1039/C39920000903.CS1 maint: uses authors parameter (link)
- Lipshutz, Bruce H.; Moretti, Robert; Crow, Robert (1990). "Mixed Higher-order Cyanocuprate-induced Epoxide Openings: 1-benzyloxy-4-penten-2-ol". Org. Synth. 69: 80. doi:10.15227/orgsyn.069.0080.
- Dietmar Seyferth (1959). "Di-n-butyldivinyltin". Org. Synth. 39: 10. doi:10.15227/orgsyn.039.0010.