Vinylcyclohexene dioxide
4-Vinylcyclohexene dioxide (VCD) is an organic compound that contains two epoxide functional groups. It is industrially used as a crosslinking agent for the production of epoxy resins.[5][6] It is a colourless liquid. It is an intermediate for synthesis of organic compounds.[2]
 | |
| Names | |
|---|---|
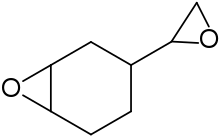
| IUPAC name
3-Oxiranyl-7-oxabicyclo[4.1.0]heptane | |
| Other names
1,2-Epoxy-4-(epoxyethyl)cyclohexane 4-Vinylcyclohexene diepoxide | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | VCD |
| ECHA InfoCard | 100.003.126 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C8H12O2 | |
| Molar mass | 140.182 g·mol−1 |
| Appearance | Colorless liquid[1] |
| Density | 1.09 g·cm−3[2][3] |
| Melting point | −108.9 °C (−164.0 °F; 164.2 K)[4] |
| Boiling point | 227 °C (441 °F; 500 K)[4] |
| Vapor pressure | 13 Pa (20 °C)[4] |
| Hazards | |
| Main hazards | Toxic |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and properties
4-Vinylcyclohexene dioxide is prepared by epoxidation of 4-vinylcyclohexene with peroxybenzoic acid.[5] Its viscosity is 15 mPa·s.[5]
Safety
4-Vinylcyclohexene dioxide, like other volatile epoxides, is classified as an alkylating agent.[5] VCD has toxic effects on fertility. It is a killer of oocytes in immature ovarian follicles in mice and rats.[7][8][9]
In pest control, it has been used as an ovotoxic agent for reducing rat fertility.[10]
gollark: 150WPMish.
gollark: Faster than that, I think?
gollark: Sometimes I plug my phone into my computer and use `scrcpy` to type unreasonably fast on it, for text conversations.
gollark: I thought it was 115. Oh no.
gollark: It's probably because something something access to paper/computers for temporary storage.
References
- Kam-Piu Ho, Wing-Leung Wong, Kin-Ming Lam, Cheuk-Piu Lai, Tak Hang Chan und Kwok-Yin Wong (2008-09-08). "A Simple and Effective Catalytic System for Epoxidation of Aliphatic Terminal Alkenes with Manganese(II) as the Catalyst". Chemistry: A European Journal. 14 (26): 7988–7996. doi:10.1002/chem.200800759. PMID 18618538.CS1 maint: multiple names: authors list (link)
- Kh. M. Alimardanov, O. A. Sadygov, N. I. Garibov und M. Ya. Abdullaeva (2012-11-07). "Liquid-phase synthesis of cyclic diene diepoxides using metal halides and hydrogen peroxide". Russian Journal of Organic Chemistry. 48 (10): 1302–1308. doi:10.1134/S1070428012100077.CS1 maint: multiple names: authors list (link)
- L. A. Mukhamedova, G. Kh. Gil'manova, M. I. Kudryavtseva, F. G. Nasybullina und A. S. Kireeva (July 1982). "Synthesis and testing of the antiviral activity of epoxy and triazo derivatives of cyclohexane". Pharmaceutical Chemistry Journal. 16 (7): 510–514. doi:10.1007/BF00761540.CS1 maint: multiple names: authors list (link)
- Record of CAS RN 106-87-6 in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 24. März 2015.
- Pham, Ha Q.; Marks, Maurice J. (2005). "Epoxy Resins". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a09_547.pub2. ISBN 3527306730.
- US 2555500 "Copolymers of 4-vinylcyclohexene dioxide."
- Kappeler, Connie J.; Hoyer, Patricia B. (2012-02-01). "4-vinylcyclohexene diepoxide: a model chemical for ovotoxicity". Systems Biology in Reproductive Medicine. 58 (1): 57–62. doi:10.3109/19396368.2011.648820. ISSN 1939-6376. PMC 3307534. PMID 22239082.
- Takai, Yasushi; Canning, Jacqueline; Perez, Gloria I.; Pru, James K.; Schlezinger, Jennifer J.; Sherr, David H.; Kolesnick, Richard N.; Yuan, Junying; Flavell, Richard A. (2003-01-01). "Bax, caspase-2, and caspase-3 are required for ovarian follicle loss caused by 4-vinylcyclohexene diepoxide exposure of female mice in vivo". Endocrinology. 144 (1): 69–74. doi:10.1210/en.2002-220814. ISSN 0013-7227. PMID 12488331.
- Hoyer, P. B.; Devine, P. J.; Hu, X.; Thompson, K. E.; Sipes, I. G. (2001-02-01). "Ovarian toxicity of 4-vinylcyclohexene diepoxide: a mechanistic model". Toxicologic Pathology. 29 (1): 91–99. doi:10.1080/019262301301418892. ISSN 0192-6233. PMID 11215690.
- Hoyer PB1, Devine PJ, Hu X, Thompson KE, Sipes IG (Jan–Feb 2001). "Ovarian toxicity of 4-vinylcyclohexene diepoxide: a mechanistic model". Toxicol Pathol. 29 (1): 91–99. doi:10.1080/019262301301418892. PMID 11215690.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.