Vinylacetylene
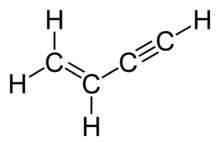
Vinylacetylene is the organic compound with the formula C4H4. The colourless gas was once used in the polymer industry. It is composed of both alkyne and alkene groups and is the simplest enyne.
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
But-1-en-3-yne | |
| Other names
Butenyne 3-Butene-1-yne | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.650 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H4 | |
| Molar mass | 52.07456 g/mol |
| Appearance | colourless gas |
| Boiling point | 0 to 6 °C (32 to 43 °F; 273 to 279 K) |
| low | |
| Hazards | |
| Main hazards | flammable |
| NFPA 704 (fire diamond) | |
| Flash point | < −5 °C (23 °F; 268 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Vinylacetylene is extremely dangerous because in high enough concentrations (typically > 30 mole percent, but pressure dependent) it can auto-detonate (explode without air being present) especially at elevated pressures, such as those seen in chemical plants processing C4 hydrocarbons.[2] An example of such an explosion occurred at a Union Carbide plant in Texas City in 1969.[3]
Synthesis
Vinylacetylene was first synthesized by Hofmann elimination of the related quaternary ammonium salt:[4]
- [(CH3)3NCH2CH=CHCH2N(CH3)3]I2 → 2 [(CH3)3NH]I + HC≡C-CH=CH2
It is usually synthesized by dehydrohalogenation of 1,3-dichloro-2-butene.[5] It also arises via the dimerization of acetylene or dehydrogenation of 1,3-butadiene.
Application
At one time, chloroprene (2-chloro-1,3-butadiene), an industrially important monomer, was produced via the intermediacy of vinyl acetylene.[6] In this process, acetylene is dimerized to give vinyl acetylene, which is then combined with hydrogen chloride to give 4-chloro-1,2-butadiene via 1,4-addition. This allene derivative which, in the presence of cuprous chloride, rearranges to 2-chloro-1,3-butadiene:[7]
- H2C=CH-C≡CH + HCl → H2ClC-CH=C=CH2
- H2ClC-CH=C=CH2 → H2C=CH-CCl=CH2
References
- http://www.newenv.com/resources/nfpa_chemicals
- Ritzert and Berthol, Chem Ing Tech 45(3), 131-136, Feb 1973, reproduced in Viduari, J Chem Eng Data 20(3), 328-333, 1975.
- Carver, Chemical Process Hazards V, Paper F
- Richard Willstätter, Theodor Wirth "Über Vinyl-acetylen" Ber., volume 46, p. 535 (1913). doi:10.1002/cber.19130460172
- G. F. Hennion, Charles C. Price, Thomas F. McKeon, Jr. (1958). "Monovinylacetylene". 38: 70. doi:10.15227/orgsyn.038.0070. Cite journal requires
|journal=(help)CS1 maint: multiple names: authors list (link) - Wallace H. Carothers, Ira Williams, Arnold M. Collins, and James E. Kirby (1937). "Acetylene Polymers and their Derivatives. II. A New Synthetic Rubber: Chloroprene and its Polymers". J. Am. Chem. Soc. 53 (11): 4203–4225. doi:10.1021/ja01362a042.CS1 maint: multiple names: authors list (link)
- Manfred Rossberg, Wilhelm Lendle, Gerhard Pfleiderer, Adolf Tögel, Eberhard-Ludwig Dreher, Ernst Langer, Heinz Rassaerts, Peter Kleinschmidt, Heinz Strack, Richard Cook, Uwe Beck, Karl-August Lipper, Theodore R. Torkelson, Eckhard Löser, Klaus K. Beutel, “Chlorinated Hydrocarbons” in Ullmann’s Encyclopedia of Industrial Chemistry, 2006 John Wiley-VCH: Weinheim.doi: 10.1002/14356007.a06_233.pub2
