Veratraldehyde
Veratraldehyde (3,4-dimethoxybenzaldehyde) is an organic compound that is widely used as a flavorant and odorant. The compound is structurally related to benzaldehyde.
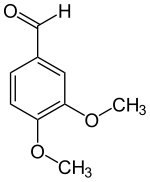 | |
| Names | |
|---|---|
| Preferred IUPAC name
3,4-Dimethoxybenzaldehyde | |
| Systematic IUPAC name
3,4-Dimethoxybenzenecarbaldehyde | |
| Other names
Methylvanillin; Veratric aldehyde; Veratral; Veratryl aldehyde; Veratrum aldehyde; Vanillin methyl ether | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.976 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H10O3 | |
| Molar mass | 166.176 g·mol−1 |
| Appearance | Peach coloured crystals |
| Density | 1.114 g/mL |
| Melting point | 40 to 43 °C (104 to 109 °F; 313 to 316 K) |
| Boiling point | 281 °C (538 °F; 554 K) |
| organic solvents | |
| Hazards | |
| Main hazards | Harmful |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
This compound is popular commercially because of its pleasant woody fragrance. It is derivative of vanillin, from which it is prepared by methylation.[1]
Uses
Veratraldehyde can be used as an intermediate in the synthesis of some pharmaceutical drugs including amiquinsin, hoquizil, piquizil, prazosin, quinazocin, tiapamil, toborinone, verazide, and vetrabutine.
gollark: They are, though.
gollark: Maybe your software is just wrong, instead of right.
gollark: Probably.
gollark: You can use 7zip or GNU `tar` or something.
gollark: Look, it isn't my fault if you can't open TAR files.
See also
References
- Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, , Kurt Bauer, Dorothea Garbe and Horst Surburg "Flavors and Fragrances" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2003. doi:10.1002/14356007.a11_141
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.