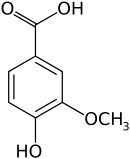
Vanillic acid
Vanillic acid (4-hydroxy-3-methoxybenzoic acid) is a dihydroxybenzoic acid derivative used as a flavoring agent. It is an oxidized form of vanillin. It is also an intermediate in the production of vanillin from ferulic acid.[2][3]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
4-Hydroxy-3-methoxybenzoic acid | |||
| Other names
4-Hydroxy-m-anisic acid, Vanillate | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.061 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C8H8O4 | |||
| Molar mass | 168.148 g·mol−1 | ||
| Appearance | White to light yellow powder or crystals | ||
| Melting point | 210 to 213 °C (410 to 415 °F; 483 to 486 K) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Related compounds |
Vanillin, vanillyl alcohol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Occurrence in nature
The highest amount of vanillic acid in plants known so far is found in the root of Angelica sinensis,[4] an herb indigenous to China, which is used in traditional Chinese medicine.
Metabolism
Vanillic acid is one of the main catechins metabolites found in humans after consumption of green tea infusions.[7]
Uses
Vanillic acid is used in the synthesis of:
- the analeptic drug etamivan.[9]
- Modecainide
- Vanillic acid is acetylated and converted to its acid chloride; S.B. amidation with bromhexine gives Brovanexine.[10]
- Vanitiolide
- Vanyldisulfamide (need synthesis still).
gollark: Do you agree?
gollark: I'd like to propose the Bees Theorem: any application's bloat can be increased by appending "bees" in a comment to the end of its source code.
gollark: Interesting!
gollark: Idea: make a language which is maximally bloated according to Nobody.
gollark: Hmm, we should probably procedurally generate them somehow.
References
- "Vanillic acid (4-hydroxy-3-methoxybenzoic acid)". chemicalland21.com. Retrieved 2009-01-28.
- Lesage-Meessen L, Delattre M, Haon M, Thibault JF, Ceccaldi BC, Brunerie P, Asther M (October 1996). "A two-step bioconversion process for vanillin production from ferulic acid combining Aspergillus niger and Pycnoporus cinnabarinus". J. Biotechnol. 50 (2–3): 107–113. doi:10.1016/0168-1656(96)01552-0. PMID 8987621.
- Civolani C, Barghini P, Roncetti AR, Ruzzi M, Schiesser A (June 2000). "Bioconversion of ferulic acid into vanillic acid by means of a vanillate-negative mutant of Pseudomonas fluorescens strain BF13". Appl. Environ. Microbiol. 66 (6): 2311–2317. doi:10.1128/AEM.66.6.2311-2317.2000. PMC 110519. PMID 10831404.
- Duke, JA (1992). Handbook of phytochemical constituents of GRAS herbs and other economic plants. CRC Press, 999 edition. ISBN 978-0-8493-3865-6. Archived from the original on 2015-09-23. Retrieved 2012-01-07.
- Pacheco-Palencia LA, Mertens-Talcott S, Talcott ST (Jun 2008). "Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Açaí (Euterpe oleracea Mart.)". J Agric Food Chem. 56 (12): 4631–4636. doi:10.1021/jf800161u. PMID 18522407.
- Gálvez, Miguel Carrero; Barroso, Carmelo García; Pérez-Bustamante, Juan Antonio (1994). "Analysis of polyphenolic compounds of different vinegar samples". Zeitschrift für Lebensmittel-Untersuchung und -Forschung. 199: 29–31. doi:10.1007/BF01192948.
- Pietta, P. G.; Simonetti, P.; Gardana, C.; Brusamolino, A.; Morazzoni, P.; Bombardelli, E. (1998). "Catechin metabolites after intake of green tea infusions". BioFactors. 8 (1–2): 111–8. doi:10.1002/biof.5520080119. PMID 9699018.
- Lim M, Yoon CM, An G, Rhee H (2007). Tetrahedron Lett. 48 (22): 3835–3839. Missing or empty
|title=(help) - Kvasnicka Erich, Kratzl Karl U.S. Patent 2,641,612 (1952 to Chemie Linz AG).
- GB1432904A
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.