Valence and conduction bands
In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level and thus determine the electrical conductivity of the solid. In non-metals, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature, while the conduction band is the lowest range of vacant electronic states. On a graph of the electronic band structure of a material, the valence band is located below the Fermi level, while the conduction band is located above it.
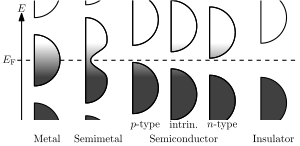
The distinction between the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands.
Band gap
In semiconductors and insulators the two bands are separated by a band gap, while in semimetals the bands overlap. A band gap is an energy range in a solid where no electron states can exist due to the quantization of energy. Electrical conductivity of non-metals is determined by the susceptibility of electrons to be excited from the valence band to the conduction band.
Electrical conductivity
Semiconductor band structure
See electrical conduction and semiconductor for a more detailed description of band structure.
In solids, the ability of electrons to act as charge carriers depends on the availability of vacant electronic states. This allows the electrons to increase their energy (i.e., accelerate) when an electric field is applied. Similarly, holes (empty states) in the almost filled valence band also allow for conductivity.
As such, the electrical conductivity of a solid depends on its capability to flow electrons from the valence to the conduction band. Hence, in the case of a semimetal with an overlap region, the electrical conductivity is high. If there is a small band gap (Eg), then the flow of electrons from valence to conduction band is possible only if an external energy (thermal, etc.) is supplied; these groups with small Eg are called semiconductors. If the Eg is sufficiently high, then the flow of electrons from valence to conduction band becomes negligible under normal conditions; these groups are called insulators.
There is some conductivity in semiconductors, however. This is due to thermal excitation—some of the electrons get enough energy to jump the band gap in one go. Once they are in the conduction band, they can conduct electricity, as can the hole they left behind in the valence band. The hole is an empty state that allows electrons in the valence band some degree of freedom.
See also
- Electrical conduction for more information about conduction in solids, and another description of band structure.
- Fermi sea
- Semiconductor for a full explanation of the band structure of materials.
- HOMO/LUMO
- Valleytronics
References
- "Chembio".
- "Hyperphysics".
- Kittel, Charles (2005). Introduction to Solid State Physics. Wiley. ISBN 0-471-41526-X.