Uroerythrin
Uroerythrin is a red pigment present in the urine, where it is part of a group of yellow, brown and red pigments generally designated as urochrome.[2]
 | |
| Names | |
|---|---|
| IUPAC name
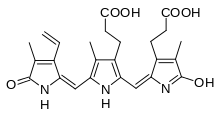
1,14,15,17-tetrahydro-2,7,13-trimethyl-1,14-dioxo-3-vinyl-16H-tripyrrin-8,12-dipropionic acid[1] | |
| Other names
Biotripyrrin-a | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C 25H 27N 3O 6 | |
| Molar mass | 465.498 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Urinary pigments
Pigments excreted in urine are partially absorbed by urate sediments ( sedimentum latrerium ), which consists of cell debris and sedimented urinary components formed when the acidified urine is stored below room temperature.[2] These urate sediments looks reddish or pink due to the presence of a main pigment first introduced by Simons in 1842 as uroerythrin,[3]
Clinical significance
From early clinical observations it is known that uroerythrin is present in every urine and increased amounts are observed in pathological states, e.g. metabolic disorders with high fever or tissue degradation.[4]
Chemical structure
The chemical structure of most of urochromes is still unknown, since they are very labile pigments that are easily decomposed in the light. In particular uroerythrin, remains unresolved until 1975 with previous papers that describes it like a peptidic compound.[5][6][7] In 1975 its structure was described based on mass spectrometry, infrared spectroscopy and nuclear magnetic resonance.[2]
References
- Xiang H. Wang (2008). "Medical Use of Bilirubin and its Structural Analogues".
- Josef BERUTER; Jean-Pierre COLOMBO & Urs Peter SCHLUNEGGER (1975). "Isolation and Identification of the Urinary Pigment Uroerythrin". Eur. J. Biochem. 56: 239–244. doi:10.1111/j.1432-1033.1975.tb02226.x.
- Simon, J.F. (1842). Anthropochemie: 343. Missing or empty
|title=(help) - Proust, L. (1800). Ann. Chem. (36): 265–269. Missing or empty
|title=(help) - Minder, W. (1970) Doctoral thesis, University of Berne, CH
- Hansen, S. E. (1969) Acta Chenz. Scand. 23, 3461-3465
- Hansen, S. E. (1969) Acta Chenz. Scand. 23, 3466-3472