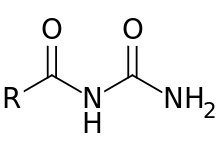
Acylurea
Acylureas (also called N-acylureas or ureides) are a class of chemical compounds formally derived from the acylation of urea.[1]
Uses
Insecticides
A subclass of acylureas known as benzoylureas are insecticides. They act as insect growth regulators by inhibiting the synthesis of chitin resulting in weakened cuticles and preventing molting.[2] Members of this class include diflubenzuron, flufenoxuron, hexaflumuron, lufenuron, and teflubenzuron.
Anticonvulsants and sedatives
The acylurea functional group is also found in some pharmaceutical drugs such as the anticonvulsants phenacemide, pheneturide, chlorphenacemide, and acetylpheneturide (which are phenylureides),[3] and the sedatives acecarbromal, bromisoval, and carbromal (which are bromoureides). Others include apronal (apronalide), capuride, and ectylurea. As the barbiturates are basically cyclic ureas, these drugs are structurally and mechanistically related to them.[4] The phenylureides are also closely related to the hydantoins, such as phenytoin, and may be considered ring-opened analogues of them.[5]
Related
- Diureides
A diureide is a complex nitrogenous substance regarded as containing two molecules of urea or their radicals, e.g. uric acid or allantoin.
- Hydantoins
Hydantoin, or glycolylurea, can be considered the cyclic form of acylurea.
References
- "N-acylurea". European Molecular Biology Laboratory.
- Vincent H. Resh and Ring T. Cardé, ed. (2009). Encyclopedia of Insects. Academic Press. p. 157.
- Hans-Hasso Frey; D. Janz (6 December 2012). Antiepileptic Drugs. Springer Science & Business Media. pp. 601–. ISBN 978-3-642-69518-6.
- David A. Williams; William O. Foye; Thomas L. Lemke (January 2002). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 380–. ISBN 978-0-683-30737-5.
- Dr. S. S. Kadam (1 July 2007). PRINCIPLES OF MEDICINAL CHEMISTRY Vol. - II. Pragati Books Pvt. Ltd. pp. 147–. ISBN 978-81-85790-03-9.