Unresolved complex mixture
Unresolved complex mixture (UCM), or hump, is a feature frequently observed in gas chromatographic (GC) data of crude oils and extracts from organisms exposed to oil.[1]
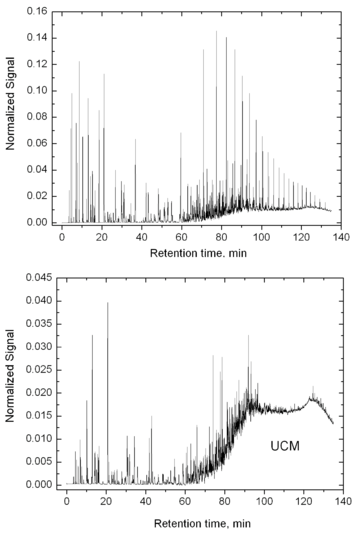
The reason for the UCM hump appearance is that GC cannot resolve and identify a significant part of the hydrocarbons in crude oils. The resolved components appear as peaks while the UCM appears as a large background/platform. In non-biodegraded oils the UCM may comprise less than 50% of the total area of the chromatogram, while in biodegraded oils this figure can rise to over 90%. UCMs are also observed in certain refined fractions such as lubricating oils [1] and references therein.
One reason why it is important to study the nature of UCMs is that some have been shown to contain toxic components,[2][3][4][5][6][7][8][9][10] but only a small range of known petrogenic toxicants, such as the USEPA list of 16 polycyclic aromatic hydrocarbons (PAHs), tend to be routinely monitored in the environment.
Analysis of the hydrocarbon fraction of crude oils by gas chromatography (GC) reveals a complex mixture containing many thousands of individual components.[11] Components that are resolved by GC have been extensively studied e.g.[12] However, despite the application of many analytical techniques the remaining components have, until very recently, proved difficult to separate due to the large numbers of co-eluting compounds. Gas chromatograms of mature oils have prominent n-alkane peaks which distract attention from the underlying unresolved complex mixture (UCM) of hydrocarbons often referred to as the ‘hump’. Processes such as weathering and biodegradation result in a relative enrichment of the UCM component by removal of resolved components and the creation of new compounds.[13] It has been shown that both resolved and unresolved components of oils are subject to concurrent biodegradation,[1] i.e. it is not a sequential process, but due to the recalcitrant nature of some components, the rates of biodegradation of individual compounds greatly varies. The UCM fraction often represents the major component of hydrocarbons within hydrocarbon-polluted sediments [5] (see reference therein) and biota e.g.[2][3][14][15] A number of studies has now demonstrated that aqueous exposure to components within the UCM can affect the health of marine organisms,[2][3][4][5][6][7][8] including possible hormonal disruption,[9] and high concentrations of environmental UCMs have been strongly implicated with impaired health in wild populations.[4][7][16][17]
Weathering and biodegradion of oils within the marine environment
Environmental UCMs result from highly degraded petroleum hydrocarbons and once formed they can stay largely unchanged in sediments for many years. For example, in 1969 a diesel oil spill contaminated saltmarsh sediment within Wild Harbor River, US; by 1973 only a baseline hump was observed, which remained largely unchanged within the anaerobic sediment for 30.[18] In a study of the potential for UCM-dominated oil to be further degraded, it was concluded that even using bacteria specifically adapted for complex UCM hydrocarbons in conjunction with nutrient enrichment, biodegradation rates would still be relatively slow.[19] Bacterial degradation of hydrocarbons is complex and will depend on environmental conditions (e.g. aerobic or anaerobic, temperature, nutrient availability, available species of bacteria etc.).
Analysis of UCM hydrocarbons
A relatively recent analytical tool that has been used for the separation of UCMs is comprehensive two-dimensional GC (GC×GC) . This powerful technique, introduced by Liu and Phillips [20] combines two GC columns with different separation mechanisms: typically a primary column that separates compounds based on volatility coupled to a second short column that separates by polarity. The two columns are connected by a modulator, a device that traps, focuses and re-injects the peaks that elute from the first column into the second column. Each peak eluting from the first column (which may be a number of co-eluting peaks) is further separated on the second column. The second separation is rapid, allowing the introduction of subsequent fractions from the first column without mutual interference. Dallüge et al.[21] reviewed the principles, advantages and main characteristics of this technique. One of the main advantages is the very high separation power, making the technique ideal for unravelling the composition of complex mixtures. Another important feature of GC×GC is that chemically related compounds show up as ordered structures within the chromatograms, i.e. isomers appear as distinct groups in the chromatogram as a result of their similar interaction with the second dimension column phase.[22] The use of GC×GC for the characterization of complex petrochemical mixtures has been extensively reviewed.[23] Most research into petrochemical hydrocarbons using GC×GC has utilised flame ionisation detection (FID) but mass spectrometry (MS) is necessary to obtain the structural information necessary to identify unknown compounds. Currently, only time-of-flight MS (ToF-MS) can deliver the high acquisition rates required to analyse GC×GC.
Toxicity of UCM hydrocarbon components
There is compelling evidence that components within some UCMs are toxic to marine organisms. The clearance rate (also known as feeding feed) of mussels was reduced by 40% following exposure to a monoaromatic UCM derived from a Norwegian crude oil.[10] The toxicity of monoaromatic UCM components was further evidenced by an elegant set of experiments using transplantations of clean and polluted mussels.[3] Recent analysis by GC×GC-ToF-MS of UCMs extracted from the mussel tissues, has shown that they contain a vast array of both known and unknown compounds.[4] The comparative analysis of UCMs extracted from mussels known to possess high, moderate and low Scope for Growth (SfG), a measure of the capacity for growth and reproduction,[24] revealed that branched alkylbenzenes represented the largest structural class within the UCM of mussels with low SfG; also, branched isomers of alkyltetralins, alkylindans and alkylindenes were prominent in the stressed mussels.[4] Laboratory toxicity tests using both commercially available and specially synthesised compounds revealed that such branched alkylated structures were capable of producing the observed poor health of the mussels.[4][7] The reversible effects observed in mussels following exposure to the UCM hydrocarbons identified to date are consistent with non-specific narcosis (also known as baseline) mode of action of toxicity.[6] There is no evidence that toxic UCM components can biomagnify through the food chain. Crabs (Carcinus maenas) that were fed a diet of mussels contaminated with environmentally realistic concentrations of branched alkylbenzenes, suffered behavioural disruption but only a small concentration of the compounds were retained in the midgut of the crabs.[8] Within marsh sediments still contaminated with high concentrations of UCM hydrocarbons from the Florida barge oil spill in 1969 (see above,) the behaviour and feeding of fiddler crabs (Uca pugnax) was reported to be affected.[25]
Polar UCMs
Much of the past research into the composition and toxicity of UCM hydrocarbons has been conducted by the Petroleum and Environmental Geochemistry Group (PEGG) at the University of Plymouth, UK. As well as the hydrocarbon UCM, oils also contain more polar compounds such as those containing oxygen, sulphur or nitrogen. These compounds can be very soluble in water and hence bioavailable to marine and aquatic organisms. Polar UCMs are present within produced waters from oil rigs and from oil sands processing. A polar UCM fraction extracted from North Sea oil produced water was reported to elicit hormonal disruption by way of both estrogen receptor agonist and androgen receptor agonist activity.[9] Ongoing concern regarding the potential toxicity of components within Athabasca Oil Sands (Canada) tailings ponds has highlighted the need for identification of the compounds present. Until recently, such positive identification of individual so-called naphthenic acids from oil sands produced waters had so far eluded characterisation but recent research by PEGG presented at a SETAC conference in 2010 [26] revealed that, using a new GCxGC-TOF-MS, it was possible to resolve and identify a range of new compounds within such highly complex extracts. One group of compounds found to be present were tricyclic diamondoid acids.[27] These structures had previously not even been considered as naphthenic acids and suggests an unprecedented degree of biodegradation of some of the oil in the oil sands.
See also
- Gas-liquid chromatography
- Ecotoxicology
- Environmental chemistry
- Toxicology
- Pollution
- Endocrine disruptor
References
- Gough, M. A. & Rowland, S. J. Characterization of Unresolved Complex-Mixtures of Hydrocarbons in Petroleum. Nature 344, 648-650 (1990).
- Rowland, S., Donkin, P., Smith, E. & Wraige, E. Aromatic hydrocarbon "humps" in the marine environment: Unrecognized toxins? Environmental Science & Technology 35, 2640-2644 (2001).
- Donkin, P., Smith, E. L. & Rowland, S. J. Toxic effects of unresolved complex mixtures of aromatic hydrocarbons accumulated by mussels, Mytilus edulis, from contaminated field sites. Environmental Science & Technology 37, 4825-4830 (2003).
- Booth, A. M. et al. Unresolved Complex Mixtures of Aromatic Hydrocarbons: Thousands of Overlooked Persistent, Bioaccumulative, and Toxic Contaminants in Mussels. Environ. Sci. Technol. 41, 457-464 (2007).
- Scarlett, A., Galloway, T. S. & Rowland, S. J. Chronic toxicity of unresolved complex mixtures (UCM) of hydrocarbons in marine sediments Journal of Soils & Sediments 7, 200-206 (2007).
- Scarlett, A., Rowland, S. J., Galloway, T. S., Lewis, A. C. & Booth, A. M. Chronic sublethal effects associated with branched alkylbenzenes bioaccumulated by mussels. Environmental Toxicology and Chemistry 27, 561-567 (2008).
- Booth, A., Scarlett, A., Lewis, C. A., Belt, S. T. & Rowland, S. J. Unresolved Complex Mixtures (UCMs) of Aromatic Hydrocarbons: Branched Alkyl Indanes and Branched Alkyl Tetralins are present in UCMs and accumulated by and toxic to, the mussel Mytilus edulis. Environ Sci Technol. 42, 8122-8126 (2008).
- Scarlett, A., Dissanayake, A., Rowland, S. J. & Galloway, T. S. Behavioral, physiological, and cellular responses following trophic transfer of toxic monoaromatic hydrocarbons. Environmental Toxicology and Chemistry 28, 381-387 (2009).
- Tollefsen, K. E., Harman, C., Smith, A. & Thomas, K. V. Estrogen receptor (ER) agonists and androgen receptor (AR) antagonists in effluents from Norwegian North Sea oil production platforms. Marine Pollution Bulletin 54, 277-283 (2007).
- Smith, E., Wraige, E., Donkin, P. & Rowland, S. Hydrocarbon humps in the marine environment: Synthesis, toxicity, and aqueous solubility of monoaromatic compounds. Environmental Toxicology and Chemistry 20, 2428-2432 (2001).
- Sutton, P. A., Lewis, C. A. & Rowland, S. J. Isolation of individual hydrocarbons from the unresolved complex hydrocarbon mixture of a biodegraded crude oil using preparative capillary gas chromatography. Organic Geochemistry 36, 963-970 (2005).
- Killops, S. D. & Killops, V. J. An introduction to organic geochemistry (Longman, Harlow, England, 1993).
- Peters, K. E., Walters, C. C. & Moldowan, J. M. The biomarker guide: Volume 1, Biomarkers and Isotopes in the Environment and Human History (Cambridge University Press, Cambridge, England, 2005).
- Fowler, S. W., Readman, J. W., Oregioni, B., Villeneuve, J. P. & McKay, K. Petroleum-Hydrocarbons and Trace-Metals in Nearshore Gulf Sediments and Biota before and after the 1991 War - an Assessment of Temporal and Spatial Trends. Marine Pollution Bulletin 27, 171-182 (1993).
- Colombo, J. C. et al. Oil spill in the Rio de la Plata estuary, Argentina: 1. Biogeochemical assessment of waters, sediments, soils and biota. Environmental Pollution 134, 277-289 (2005).
- Crowe, T. P., Smith, E. L., Donkin, P., Barnaby, D. L. & Rowland, S. J. Measurements of sublethal effects on individual organisms indicate community-level impacts of pollution. Journal of Applied Ecology 41, 114-123 (2004).
- Guerra-Garcia, J. M., Gonzalez-Vila, F. J. & Garcia-Gomez, J. C. Aliphatic hydrocarbon pollution and macrobenthic assemblages in Ceuta harbour: a multivariate approach. Marine Ecology Progress Series 263, 127-138 (2003).
- Reddy, C. M. et al. The West Falmouth oil spill after thirty years: the persistence of petroleum hydrocarbons in marsh sediments. Environmental Science & Technology 36, 4754-4760 (2002).
- McGovern, E. (Marine Institute Fisheries Research Centre, Dublin, 1999).
- Liu, Z. Y. & Phillips, J. B. Comprehensive 2-Dimensional Gas-Chromatography Using an on-Column Thermal Modulator Interface. Journal of Chromatographic Science 29, 227-231 (1991).
- Dallüge, J., Beens, J. & Brinkman, U. A. T. Comprehensive two-dimensional gas chromatography: a powerful and versatile analytical tool. Journal of Chromatography A 1000, 69-108 (2003).
- Phillips, J. B. & Beens, J. Comprehensive two-dimensional gas chromatography: a hyphenated method with strong coupling between the two dimensions. Journal of Chromatography A 856, 331-347 (1999).
- Adahchour, M., Beens, J., Vreuls, R. J. J. & Brinkman, U. A. T. Recent developments in comprehensive two-dimensional gas chromatography (GC x GC) III. Applications for petrochemicals and organohalogens. Trac-Trends in Analytical Chemistry 25, 726-741 (2006).
- Widdows, J. et al. Measurement of stress effects (scope for growth) and contaminant levels in mussels (Mytilus edulis) collected from the Irish Sea. Marine Environmental Research 53, 327-356 (2002).
- Culbertson, J. B. et al. Long-term biological effects of petroleum residues on fiddler crabs in salt marshes. Marine Pollution Bulletin 54, 955-962 (2007).
- Rowland, S. J. in SETAC North America 31st Annual Meeting, Portland, U.S.A., 7–11 November 2010 (2010).
- Rowland SJ, Scarlett AG, Jones D, West CE, Frank RA. Diamonds in the Rough: Identification of Individual Naphthenic Acids in Oil Sands Process Water. Environ Sci Technol: In Press, doi:10.1021/es103721b.