Umbralisib
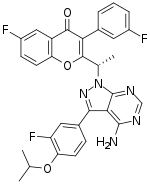
Umbralisib (development codes RP5264 and TGR 1202) is an experimental oral PI3K delta inhibitor.[1] It also inhibits CK1 epsilon.[2]
 | |
| Clinical data | |
|---|---|
| Other names | RP5264; TGR 1202 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C31H24F3N5O3 |
| Molar mass | 571.560 g·mol−1 |
| 3D model (JSmol) | |
| |
It has undergone clinical studies for chronic lymphocytic leukemia (CLL).[3][4] Three year data (including follicular lymphoma and DLBCL) was announced June 2016.[5] It is in combination trials for various leukemias and lymphomas, such as mantle cell lymphoma (MCL)[6][7] and other lymphomas.[8]
The drug has been granted the FDA's breakthrough therapy status for use in patients with marginal zone lymphoma (MZL), a type of cancer with no specifically approved therapies.[9]
References
- Clinical trial number NCT01767766 for "Evaluate the Safety and Efficacy of TGR 1202 in Patients With Relapsed or Refractory Hematologic Malignancies" at ClinicalTrials.gov
- Barrientos JC (April 2018). "Can umbralisib bring PI3Kδ out of the shadows?". The Lancet. Oncology. 19 (4): 432–434. doi:10.1016/S1470-2045(18)30154-2. PMID 29475725.
- Inman S (19 March 2016). "Novel BTK, PI3K Inhibitors on Horizon for Relapsed CLL". OncLive. Archived from the original on 1 May 2016.
- "Therapy Focus –- TG Could Benefit From Zydelig Setback". Seeking Alpha. 29 March 2016.
- "TG Therapeutics, Inc. Announces First Patient Enrolled in the Registration-Directed UNITY-DLBCL Phase 2b Trial". TG Therapeutics Inc. June 2016.
- Clinical trial number NCT02268851 for "A Phase I/Ib Safety and Efficacy Study of the PI3K-delta Inhibitor TGR-1202 and Ibrutinib in Patients With CLL or MCL" at ClinicalTrials.gov
- "Follow-Up Data for Combination of TGR-1202 (umbralisib) plus Ibrutinib in Patients with Relapsed or Refractory CLL and MCL". Globenewswire.com. 14 June 2017.
- Clinical trial number NCT02793583 for "Study to Assess the Efficacy and Safety of Ublituximab + TGR-1202 With or Without Bendamustine and TGR-1202 Alone in Patients With Previously Treated Non-Hodgkin's Lymphoma (UNITY-NHL)" at ClinicalTrials.gov
- Columbus G (22 January 2019). "FDA Grants Umbralisib Breakthrough Designation for Marginal Zone Lymphoma". OncLive. Archived from the original on 23 January 2019.
Further reading
- Barrientos JC (April 2018). "Can umbralisib bring PI3Kδ out of the shadows?". The Lancet. Oncology. 19 (4): 432–434. doi:10.1016/S1470-2045(18)30154-2. PMID 29475725.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.