Tylenchulus semipenetrans
Tylenchulus semipenetrans (Citrus nematode, Citrus root nematode) is a species of plant pathogenic nematodes and the causal agent of slow decline of citrus. T. semipenetrans is found in most citrus production areas and diverse soil textures worldwide. Their feeding strategy is semi-endoparasitic and has a very narrow host range among commonly grown crops. These nematodes are considered as major plant-parasitic nematode because they can cause 10-30% losses reported on citrus trees. They also parasitize other hosts such as olive, grape, persimmon and lilac.[1] The citrus nematode was first discovered in California in 1913 by J.R. Hodges and was later described and named by Nathan Cobb that year.[2]
| Citrus nematode | |
|---|---|
| Scientific classification | |
| Kingdom: | |
| Phylum: | |
| Class: | |
| Subclass: | |
| Order: | |
| Superfamily: | Criconematoidea |
| Family: | Tylenchulidae |
| Subfamily: | Tylenchulinae |
| Genus: | Tylenchulus |
| Species: | T. semipenetrans |
| Binomial name | |
| Tylenchulus semipenetrans | |
Morphology
T. semipenetrans is the only species of Tylenchulidae that are economically important to agriculture. Citrus nematode range from 0.25-0.35 mm long. They have an amalgamated procorpus and metacorpus, distinct isthmus, and a bulb-shaped postcorpus. They are distinct in juveniles. Both the juvenile stages and the adult male stage are vermiform in shape. The male has significantly reduced esophagus and stylet. The posterior end of the female citrus nematode becomes swollen upon feeding. She contains a single ovary and the vulva is subterminal. The female will lay up to 100 eggs deposited in a gelatinous matrix secreted from the nearby excretory pore. The pore is surrounded by small, irregularly shaped lobes; and the excretory duct is directed forward. The rectum and anus are atrophied or absent; non-functional.[3]
Life cycle and reproduction
The life cycle of the female citrus nematode is 6–8 weeks long, whereas the male citrus nematode only lives for about 7–10 days. These nematodes reproduce by amphimixis and parthenogenesis. The first-stage juvenile (J1) undergo one molt while still in the egg. The J1 has no stylet. The second-stage juveniles (J2) hatch from the eggs and the sex can be distinguished at this stage. The J2 male is short and fat. Juveniles will undergo two more molts into the J3 and J4 before becoming young adults. The citrus male nematodes are required for reproduction with females when their posterior end is exposed on the root surface. The J2 male has a stylet while the J3 and J4 have a weaker stylet. The J2 female is longer and thinner than males and they do not molt until feeding site is established. The female juveniles begin feeding ectoparasitically on epidermal root cells. It is not until the female citrus nematode becomes a young adult that she becomes the infective stage. The anterior end of the young female penetrates into the cortex of the root and begins feeding on 3-6 nurse cells. This intense feeding by the adult female will cause the posterior end to enlarge outside the root and start producing eggs. After fertilization, the female lays its eggs outside of the root in a gelatinous matrix extruded from excretory pore located near the vulva.
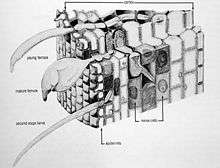
Host-parasite interaction
High population densities of the citrus nematode can result in severe damage on the citrus tree. Some above ground symptoms can be observed such as suppression of citrus tree growth, lack of vigor or decline symptoms, yellowing of foliage and small size of fruit. The young adult females penetrate into the cortex cells, become sedentary and form multiple‘nurse’ cells. The nematode feeding from these nurse cells reduces the amount of water and nutrients available to the growing plant.

For below ground symptoms, the infected roots are thicker, darker, decayed and appear dirty. This is caused by soil particles sticking the gelatinous matrices which have been excreted by the females. The infected root systems due to the nematode damage lose the ability to absorb enough water and nutrients for normal growth. Yellowing of foliage, leaf curling and dieback are consequences of insufficient root development and decayed young roots.[5]
According to Cohn 1969, 4000 juveniles/ g root are the damage threshold for slow decline disease in Israel.[6] While in California, nematicide should be applied if more than 400 nematode females/ g root are sampled in February to April or 100 females/ g root in May and June.[7] In Cyprus, the growers need to apply nematicides when the nematode densities reach 5000 juveniles/ 250 cm3 in soil.[8] Treatments are recommended when 100 females/ g root are observed in South Africa.[9] However, the age and vigor of the citrus trees, the nematode population densities in the soil, the aggressiveness of the nematodes, soil characteristics, and other environmental factors can influence the level of infestation by citrus nematode.
Management of the citrus nematode
Management practices consist of exclusion, preventive measures, and post-planting nematicide applications. All growers should avoid contaminated nursery rootstocks and use certified nematode-free soil and nematode-free rootstock (it is obligatory in some areas). Nematodes can easily be removed from seedlings by dipping the roots in 45 C water for 25 min, this kills the nematodes but does not harm the plant.[10] For cultural practices, the container-grown citrus can be treated with steam and soil solarization.[11] Fumigation and nematicides are used to reduced initial population densities. Halogenated hydro-carbons ( MBr,1-3-D and chloropicin) are the most effective.[12] Resistant rootstocks are available and this management strategy is the most useful to suppress nematode population density. Recently, the hybrid rootstock called Swingle citrumelo (Citrus paradisi x P. trifoliata) is highly resistant to the citrus nematode.[13]
References
- Verdejo-Lucas, S. and Mckenry, M.V. 2004. Management of the Citrus Nematode, Tylenchulus semipenetrans. Journal of Nematology 36: 424-432
- http://entnemdept.ufl.edu/creatures/nematode/citrus_nematode.htm
- http://www.ipm.ucdavis.edu
- Verdejo-Lucas, S.,and D.T.Kaplan. 2002. The citrus nematode: Tylenchulus semipenetrans.Pp. 207-219 in J.L. Starr, R.Cook, and J.Bridge, eds. Plant resistance to parasitic nematodes. Wallingford, UK. CAB International.
- Duncan, L.W., and E. Cohn. 1990. Nematode parasites of citrus. Pp. 321-346 in M.Luc, R.A. Sikora, and J.Bridge, eds. Plant-parasitic nematodes in subtropical and tropical agriculture. Wallingford, UK: CAB International.
- Cohn, E. 1969. The citrus nematode, Tylenchulus semipenetrans Cobb, as a pest of citrus in Israel. Proceedings of the First International Citrus Symposium Vol.2: 1013-1017.
- Westerdahl, B.B. 2000. Citrus nematodes. UC management guidelines for nematodes on citrus (http://www.ipm.ucdavis.edu).
- Philis, J. 1989. Yield loss assessment caused by the citrus nematode Tylenchulus semipenetrans on Valencia oranges in Cyprus. Nematologia Mediterranea 17:5-6.
- Le Roux, H.F.,M.C. Pretorius, and L.Huisman. 2000. Citrus nematode IPM in Southern Africa. Proceedings of the International Society of Citriculare Vol.2: 823-827.
- Verdejo-Lucas, S.,and D.T.Kaplan. 2002. The citrus nematode: Tylenchulus semipenetrans.Pp. 207-219 in J.L. Starr, R.Cook, and J.Bridge, eds. Plant resistance to parasitic nematodes. Wallingford, UK. CAB International.
- Stapleton, J.J., C.L. Elmore, and J.E. DeVay. 2000. Solarization and biofumigation also help disinfest soil. California Agriculture.54:42-45.
- Sorribas, F.J. Verdejo-Lucas, M. Galeano, J. Pastor, and C.Ornat. 2003. Effect of 1,3-dichloropropene and rootstocks alone and in combination on Tylenchulus semipenetrans and citrus tree growth in a replant managenement program. Nematropica. 34: 149-158.
- Kaplan, D.T., and J.H. O'Bannon. 1981. Evaluation and nature of citrus nematode resistance in Swingle citrumelo. Proceedings of the Florida State Horticultural Society. 94:22-36.
External links
- Nemaplex, University of California - Tylenchulus semipenetrans
- Tylenchulus semipenetrans on the UF / IFAS Featured Creatures Web site