Trypanothione
Trypanothione is an unusual form of glutathione containing two molecules of glutathione joined by a spermidine (polyamine) linker. It is found in parasitic protozoa such as leishmania and trypanosomes.[1] These protozoal parasites are the cause of leishmaniasis, sleeping sickness and Chagas' disease. Trypanothione was discovered by Alan Fairlamb. Its structure was proven by chemical synthesis.[2] It is unique to the Kinetoplastida and not found in other parasitic protozoa such as Entamoeba histolytica.[3] Since this thiol is absent from humans and is essential for the survival of the parasites, the enzymes that make and use this molecule are targets for the development of new drugs to treat these diseases.[4]
.svg.png) | |
.svg.png) Reduced form (top) and oxidized form (bottom) | |
| Names | |
|---|---|
| Other names
N1,N8-Bis(glutathionyl)spermidine | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C27H47N9O10S2 (oxidized) C27H49N9O10S2 (reduced) | |
| Molar mass | 721.84 g/mol (oxidized) 723.86 g/mol (reduced) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
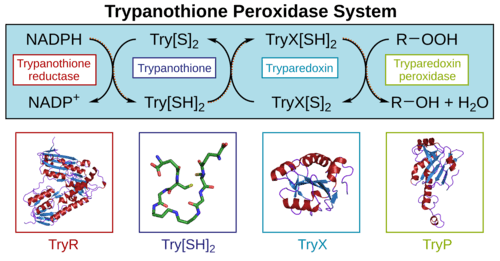
Trypanothione-dependent enzymes include reductases, peroxidases, glyoxalases and transferases. Trypanothione-disulfide reductase (TryR) was the first trypanothione-dependent enzyme to be discovered (EC 1.8.1.12). It is an NADPH-dependent flavoenzyme that reduces trypanothione disulfide. TryR is essential for survival of these parasites both in vitro and in the human host.[5][6]
A major function of trypanothione is in the defence against oxidative stress.[7] Here, trypanothione-dependent enzymes such as tryparedoxin peroxidase (TryP) reduce peroxides using electrons donated either directly from trypanothione, or via the redox intermediate tryparedoxin (TryX). Trypanothione-dependent hydrogen peroxide metabolism is particularly important in these organisms because they lack catalase. Since the trypanosomatids also lack an equivalent of thioredoxin reductase, trypanothione reductase is the sole path that electrons can take from NADPH to these antioxidant enzymes.
References
- Fairlamb AH, Cerami A (1992). "Metabolism and functions of trypanothione in the Kinetoplastida". Annu. Rev. Microbiol. 46: 695–729. doi:10.1146/annurev.mi.46.100192.003403. PMID 1444271.
- Fairlamb, A. H.; Blackburn, P.; Ulrich, P.; Chait, B. T.; Cerami, A. (Mar 1985). "Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids". Science. 227 (4693): 1485–1487. Bibcode:1985Sci...227.1485F. doi:10.1126/science.3883489. ISSN 0036-8075. PMID 3883489.
- Ariyanayagam MR, Fairlamb AH (September 1999). "Entamoeba histolytica lacks trypanothione metabolism". Mol. Biochem. Parasitol. 103 (1): 61–9. doi:10.1016/S0166-6851(99)00118-8. PMID 10514081.
- Schmidt A, Krauth-Siegel RL (November 2002). "Enzymes of the trypanothione metabolism as targets for antitrypanosomal drug development". Curr Top Med Chem. 2 (11): 1239–59. doi:10.2174/1568026023393048. PMID 12171583.
- Tovar J, Wilkinson S, Mottram JC, Fairlamb AH (July 1998). "Evidence that trypanothione reductase is an essential enzyme in Leishmania by targeted replacement of the tryA gene locus". Mol. Microbiol. 29 (2): 653–60. doi:10.1046/j.1365-2958.1998.00968.x. PMID 9720880.
- Krieger S, Schwarz W, Ariyanayagam MR, Fairlamb AH, Krauth-Siegel RL, Clayton C (February 2000). "Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress". Mol. Microbiol. 35 (3): 542–52. doi:10.1046/j.1365-2958.2000.01721.x. PMID 10672177.
- Krauth-Siegel RL, Meiering SK, Schmidt H (April 2003). "The parasite-specific trypanothione metabolism of trypanosoma and leishmania". Biol. Chem. 384 (4): 539–49. doi:10.1515/BC.2003.062. PMID 12751784.