Tropylium cation
In organic chemistry, the tropylium ion or cycloheptatrienyl cation is an aromatic species with a formula of [C7H7]+.[19] Its name derives from the molecule tropine from which cycloheptatriene (tropylidene) was first synthesized in 1881. Salts of the tropylium cation can be stable, even with nucleophiles of moderate strength e.g., tropylium tetrafluoroborate and tropylium bromide (see below). Its bromide and chloride salts[20] can be made from cycloheptatriene and bromine or phosphorus pentachloride, respectively.[21]
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2,4,6-Cycloheptatrienylium[2] | |
| Systematic IUPAC name
2,4,6-Cycloheptatrienylium[3] | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| 1902352[9] | |
| ChemSpider | |
PubChem CID |
|
| |
| Properties | |
| C7H7+[16] | |
| Molar mass | 91.132 g·mol−1 |
| Melting point | −67.41 °C (−89.34 °F; 205.74 K)[17] Mean or Weighted MP |
| Boiling point | 124.98 °C (256.96 °F; 398.13 K)[18] Adapted Stein & Brown method |
| Structure | |
| D7h | |
| regular heptagon | |
| Related compounds | |
Other anions |
Tropylium tetrafluoroborate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
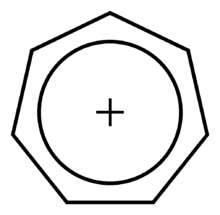
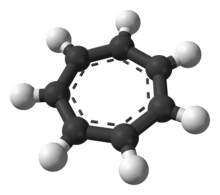
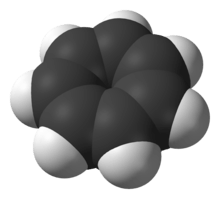
It is a regular heptagonal, planar, cyclic ion; as well, it has 6 π-electrons (4n + 2, where n = 1), which fulfills Hückel's rule of aromaticity. It can coordinate as a ligand to metal atoms.
The structure shown is a composite of seven resonance contributors in which each carbon atom carries part of the positive charge.
In 1891 G. Merling obtained a water-soluble bromine containing compound from a reaction of cycloheptatriene and bromine.[22] Unlike most hydrocarbyl bromides, this compound, later named tropylium bromide, is a water-soluble solid and is insoluble in hydrocarbons, chloroform, and ether. It is purified by crystallization from hot ethanol. Reaction with aqueous silver nitrate immediately gave a precipitate of silver bromide. The structure of tropylium bromide was deduced to be a salt, C7H7+Br–, by Doering and Knox in 1954 by analysis of its infrared and ultraviolet spectra.[23][24] The ionic structures of tropylium perchlorate (C7H7+ClO4–) and tropylium iodide (C7H7+I–) in the solid state have been confirmed by X-ray crystallography.[25] The bond length of the carbon-carbon bonds were found to be longer (147 pm) than those of benzene (140 pm) but still shorter than those of a typical single-bonded species like ethane (154 pm).
The tropylium ion is an acid in aqueous solution (i.e., an Arrhenius acid) as a consequence of its Lewis acidity: it first acts as a Lewis acid to form an adduct with water, which can then donate a proton to another molecule of water: C7H7+ + 2H2O ⇌ C7H7OH + H3O+. (Boric acid gives acidic aqueous solutions in much the same way.) The equilibrium constant is 1.8 × 10−5, making it about as acidic in water as acetic acid.[23]
Mass spectrometry
The tropylium ion is frequently encountered in mass spectrometry in the form of a signal at m/z = 91 and is used in mass spectrum analysis. This fragment is often found for aromatic compounds containing a benzyl unit. Upon ionization, the benzyl fragment forms a cation (PhCH+
2), which rearranges to the highly stable tropylium cation (C
7H+
7).
Reactions
The tropylium cation reacts with nucleophiles to form substituted cycloheptatrienes, for example:[26]
- C
7H+
7 + CN−
→ C
7H
7CN
Reduction by lithium aluminum hydride yields cycloheptatriene.[26]
Reaction with a cyclopentadienide salt of sodium or lithium yields 7-cyclopentadienylcyclohepta-1,3,5-triene:[26]
- C
7H+
7X−
+ C
5H−
5Na+
→ C
7H
7C
5H
5 + NaX
When treated with oxidising agents such as chromic acid, the tropylium cation undergoes rearrangement into benzaldehyde:[26]
- C
7H+
7 + HCrO−
4 → C
6H
5CHO + CrO
2 + H
2O
Many metal complexes of tropylium ion are known. One example is [Mo(η7-C7H7)(CO)3]+, which is prepared by hydride abstraction from cycloheptatrienemolybdenum tricarbonyl.[27]
References
- "tropylium | C7H8 | ChemSpider". www.chemspider.com. p. Names. Retrieved 30 December 2018.
tropylium
- "tropylium | C7H8 | ChemSpider". www.chemspider.com. p. Names. Retrieved 30 December 2018.
2,4,6-Cycloheptatrienylium
- "tropylium | C7H8 | ChemSpider". www.chemspider.com. p. More details. Retrieved 30 December 2018.
Systematic name 2,4,6-Cycloheptatrienylium
- "Tropylium". pubchem.ncbi.nlm.nih.gov. Retrieved 30 December 2018.
Chemical Names: Tropylium; Cycloheptatrienylium; Cyc-C7H7(+); Cyclohepta-2,4,6-trienylium
- "tropylium | C7H8 | ChemSpider". www.chemspider.com. p. Names. Retrieved 30 December 2018.
cyc-C7H7(+) cyclohepta-2,4,6-trienylium cycloheptatrienylium
- "Tropylium". pubchem.ncbi.nlm.nih.gov. Retrieved 30 December 2018.
IUPAC Name cyclohepta-1,3,5-triene
- "Tropylium". pubchem.ncbi.nlm.nih.gov. Retrieved 30 December 2018.
CAS 4118-59-6
- "Tropylium". pubchem.ncbi.nlm.nih.gov. Retrieved 30 December 2018.
CAS 4118-59-6
- "tropylium | C7H8 | ChemSpider". www.chemspider.com. Retrieved 30 December 2018.
1902352 [Beilstein]
- "tropylium | C7H8 | ChemSpider". www.chemspider.com. Retrieved 30 December 2018.
ChemSpider ID4394444
- "Tropylium". pubchem.ncbi.nlm.nih.gov. Retrieved 30 December 2018.
PubChem CID: 5224206
- "tropylium | C7H8 | ChemSpider". www.chemspider.com. p. More details. Retrieved 30 December 2018.
Std. InChIKey OJOSABWCUVCSTQ-UHFFFAOYSA-N
- "Tropylium". pubchem.ncbi.nlm.nih.gov. Retrieved 30 December 2018.
InChI InChI=1S/C7H7/c1-2-4-6-7-5-3-1/h1-7H/q+1
- "Tropylium". pubchem.ncbi.nlm.nih.gov. Retrieved 30 December 2018.
InChI Key: OJOSABWCUVCSTQ-UHFFFAOYSA-N
- "Tropylium". pubchem.ncbi.nlm.nih.gov. Retrieved 30 December 2018.
InChI Key OJOSABWCUVCSTQ-UHFFFAOYSA-N
- "Tropylium". pubchem.ncbi.nlm.nih.gov. Retrieved 30 December 2018.
Molecular Formula C7H7+
- "tropylium | C7H8 | ChemSpider". www.chemspider.com. p. Properties. Retrieved 30 December 2018.
Melting Pt (deg C): -67.41 (Mean or Weighted MP)
- "tropylium | C7H8 | ChemSpider". www.chemspider.com. p. Properties. Retrieved 30 December 2018.
Boiling Pt (deg C): 124.98 (Adapted Stein & Brown method)
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "molecule". doi:10.1351/goldbook.M04002
- A mixture of [C7H7]+Cl− and [C7H7]+[PCl6−] is produced by treatment of tropylidene with phosphorus pentachloride.
- Tropylium fluoborate Organic Syntheses, Coll. Vol. 5, p.1138 (1973); Vol. 43, p.101 (1963). link Archived 2012-08-29 at the Wayback Machine
- Merling, G. (1891). "Ueber Tropin". Berichte der Deutschen Chemischen Gesellschaft. 24 (2): 3108–3126. doi:10.1002/cber.189102402151.
- Eggers Doering, W. von; Knox, L. H. (1954). "The Cycloheptatrienylium (Tropylium) Ion". J. Am. Chem. Soc. 76 (12): 3203–3206. doi:10.1021/ja01641a027.
- Balaban, Alexandru T.; Oniciu, Daniela C.; Katritzky, Alan R. (2004). "Aromaticity as a Cornerstone of Heterocyclic Chemistry". Chem. Rev. 104 (5): 2777–2812. doi:10.1021/cr0306790.
- Kitaigorodskii, A. I.; Struchkov, Yu. T.; Khotsyanova, T. L.; Vol'pin, M. E.; Kursanov, D. N. (1960). "Crystal structures of tropylium perchlorate and iodide". Bulletin of the Academy of Sciences of the USSR Division of Chemical Science. 9 (1): 32–36. doi:10.1007/bf01178699. ISSN 0568-5230.
- O. P. Agarwai (2009). Reactions and Reagents (46th ed.). Krishna Prakashan Media. pp. 614–615. ISBN 9788187224655.
- Green, Malcolm L. H.; Ng, Dennis K. P. "Cycloheptatriene and -enyl Complexes of the Early Transition Metals" Chemical Reviews 1995, volume 95, pp. 439-73. doi:10.1021/cr00034a006