Tris(trimethylsilyl)phosphine
Tris(trimethylsilyl)phosphine is the organophosphorus compound with the formula P(SiMe3)3 (Me = methyl). It is a colorless liquid that ignites in air and hydrolyses readily.[1]
3.png) | |
| Names | |
|---|---|
| Other names
tris(trimethylsilyl)phosphane | |
| Identifiers | |
| ECHA InfoCard | 100.154.516 |
CompTox Dashboard (EPA) |
|
| Properties | |
| C9H27PSi3 | |
| Molar mass | 250.544 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.863 g/cm3 |
| Melting point | 24 °C (75 °F; 297 K) |
| Boiling point | 243–244 °C (469–471 °F; 516–517 K) |
| Hazards | |
| Main hazards | poison, inflammable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Tris(trimethylsilyl)phosphine is prepared by treating trimethylsilyl chloride, white phosphorus, and sodium-potassium alloy:[2]
- 1/4 P4 + 3 Me3SiCl + 3 K → P(SiMe3)3 + 3 KCl
Several other methods exist.[1]

Reactions
The compound hydrolyzes to give phosphine:
- P(SiMe3)3 + 3 H2O → PH3 + 3 HOSiMe3
Treatment of certain acyl chlorides with tris(trimethylsilyl)phosphine gives phosphaalkynes, one example being tert-butylphosphaacetylene.[4]
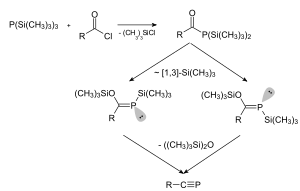 Synthesis of a phosphaalkyne using tris(trimethylsilyl)phosphine.
Synthesis of a phosphaalkyne using tris(trimethylsilyl)phosphine.
Reaction with potassium tert-butoxide cleaves one P-Si bond, giving the phosphide salt:[5]
- P(SiMe3)3 + KO-t-Bu → KP(SiMe3)2 + Me3SiO-t-Bu
It is a reagent in the preparation of metal phosphido clusters by reaction with metal halides or carboxylates. In such reactions the silyl halide or silyl carboxylate is liberated as illustrated in this idealized reaction:
- P(SiMe3)3 + 3 CuCl → Cu3P + 3 ClSiMe3
Safety
Tris(trimethylsilyl)phosphine spontaneously ignites in air, thus it is handled using air-free techniques.
References
- Kosarev, Sergey A.; Collier, Steven J. (2011). tris(trimethylsilyl)phosphin. Handbook of Reagents for Organic Synthesis: Reagents for Silicon-Mediated Organic Synthesis. pp. 422–427. doi:10.1002/047084289X.rn01332. ISBN 978-0471936237.
- Becker, Gerd; Schmidt, Helmut; Uhl, Gudrun; Uhl, Werner (1990). Tris(trimethylsilyl)phosphine and lithium bis(trimethylsilyl)phosphide·bis(tetrahydrofuran). Inorganic Syntheses. 27. pp. 243–9. doi:10.1002/9780470132586.ch48. ISBN 9780470132586.
- Fenske, D.; Holstein, W. (1994). "[Cu96P30{P(SiMe3)2}6(PEt3)18], a New Phosphorus-Bridged Copper Cluster". Angew. Chem. Int Ed. Engl. 33 (12): 1290–1292. doi:10.1002/anie.199412901.
- M. Regitz (1990). "Phosphaalkynes: new building blocks in synthetic chemistry". Chem. Rev. 90: 191–213. doi:10.1021/cr00099a007..
- Russell, Christopher A.; Townsend, Nell S. (2012). "Phosphaalkynes". In Kamer, Paul C. J.; Van Leeuwen, Piet W. N. M (eds.). Phosphorus(III) Ligands in Homogeneous Catalysis. Wiley-VCH. pp. 343–354. doi:10.1002/9781118299715.ch11. ISBN 9781118299715.