Polyquinane
A polyquinane polycyclic compound consisting of fused five-membered hydrocarbon rings.[1] If the compound is unsaturated instead of saturated, it is called a polyquinene. The simplest polyquinane is the bicyclic compound bicyclo[3.3.0]octane. Other members are triquinacene and dodecahedrane.
octane.png)
Triquinacene

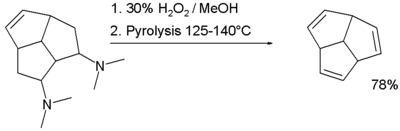
The compound triquinacene, sometimes simply called quinacene (tricyclo[5.2.1.04,10]deca-2,5,8-triene) is the second member of a family of polyquinenes. It was synthesized in 1964 in the group of R. B. Woodward[2] in connection with its suspected homoaromatic properties—though it was found to have no such properties—and also as part of a failed attempt to synthesize the then-elusive compound dodecahedrane. Triquinacene is stable, and has a melting point of 18 °C. The final step of its synthesis is a double Cope reaction to form two of the three alkenes.
See also
- Fused 6 aromatic membered rings: the acenes
- Triquinacene is isomeric with: bullvalene, diisopropenyldiacetylene
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "polyquinanes (polyquinenes)". doi:10.1351/goldbook.P04751
- Woodward, R. B.; Fukunaga, T.; Kelly, Robert C. (1964). "Triquinacene". Journal of the American Chemical Society. 86 (15): 3162–3164. doi:10.1021/ja01069a046.