1,3,5-Triazido-2,4,6-trinitrobenzene
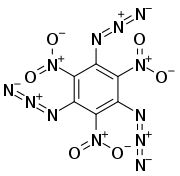
1,3,5-Triazido-2,4,6-trinitrobenzene, also known as TATNB (triazidotrinitrobenzene) and TNTAZB (trinitrotriazidobenzene), is an aromatic high explosive composed of a benzene ring with three azido groups (-N3) and three nitro groups (-NO2) alternating around the ring, giving the chemical formula C6(N3)3(NO2)3. Its velocity of detonation is 7,350 meters per second, comparable to TATB (triaminotrinitrobenzene).
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,3,5-Triazido-2,4,6-trinitrobenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6N12O6 | |
| Molar mass | 336.144 g·mol−1 |
| Appearance | yellow crystals[1] |
| Structure[1] | |
| monoclinic | |
| P21/c, No. 14 | |
| Explosive data | |
| Detonation velocity | 7350 m/s |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
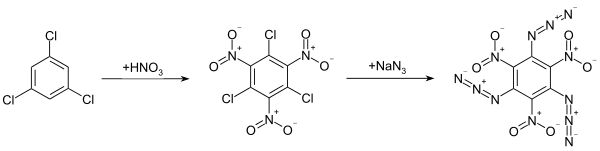
The compound was first synthesized in 1924 by Oldřich Turek.[2] It can be prepared by the reaction of 1,3,5--trichloro-2,4,6-trinitrobenzene with sodium azide, which is obtained from the nitration of 1,3,5-trichlorobenzene with nitric acid and sulfuric acid.[2]
Another route uses the nitration of 1,3,5-triazido-2,4-dinitrobenzene.[1]
Properties
Physical properties
1,3,5-Triazido-2,4,6-trinitrobenzene forms yellow crystalline solids that melt at 131 °C.[3] The crystals are monoclinic with space group P21/c. Its formation is highly endothermic, with an enthalpy of formation of 765.8 kJ/mol and an enthalpy of combustion of 3200 kJ/mol.[1]
Chemical Properties
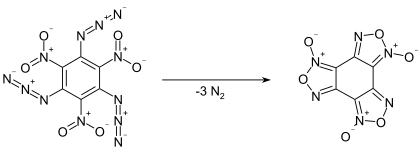
Even at low temperatures, the compound slowly decomposes by giving off nitrogen gas, converting into benzotrifuroxan. This reaction proceeds quantitatively within 14 hours at 100 °C.[2] As a solution in m-xylene, first order kinetics were observed for the decomposition, with a half-life of 340 minutes at 70 °C, 89 minutes at 80 °C, and 900 seconds at 100 °C.[4]
The compound explodes if rapidly heated above 168 °C.[1]
References
- Adam, David; Karaghiosoff, Konstantin; Klapötke, Thomas M.; Holl, Gerhard; Kaiser, Manfred (2002). "Triazidotrinitro Benzene: 1,3,5‐(N3)3‐2,4,6‐(NO2)3C6". Propellants Explos. Pyrotech. 27 (1). doi:10.1002/1521-4087%28200203%2927%3A1<7%3A%3AAID-PREP7>3.0.CO%3B2-J.
- Matyáš, Robert; Pachman, Jiří (2013). Primary Explosives. Springer Science & Business Media. pp. 118–121. ISBN 9783642284366.
- Burov, Yu. M.; Nazin, G. M.; Manelis, G. B. (1999). "Retardation of Monomolecular Reactions in the Solid Phase". Russian Chemical Bulletin. 48 (7): 1250–1254.
- Korsunskii, B. L.; Apina, T. A. (1971). "Kinetics of the Thermal Decomposition of 1,3,5-Triazido-2,4,6-trinitrobenzene in Solution". Bulletin of the Academy of Sciences of the USSR, Division of Chemical Science. 20 (9): 1971–1973. doi:10.1007/BF00854439.