Trimethylhexamethylenediamine
Trimethylhexamethylenediamine is the name used to refer to a mixture of two isomers of trimethyl-1,6-hexanediamine. The mixture is used as a monomer in nylon TMDT. It is available commercially under the trade name Vestamin TMD from the company Evonik Industries.
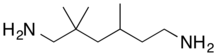 | |
| Names | |
|---|---|
| IUPAC name
2,2,4-Trimethyl-hexane-1,6-diamine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| |
| |
| Properties | |
| C9H22N2 | |
| Molar mass | 158.289 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
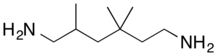 | |
| Names | |
|---|---|
| IUPAC name
2,4,4-Trimethyl-1,6-hexanediamine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| |
| |
| Properties | |
| C9H22N2 | |
| Molar mass | 158.289 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trimethylhexamethylenediamine is synthesized from isophorone.[2] Isophorone is reduced by hydrogenation to the trimethylcyclohexanol, which is then oxidized with nitric acid (in the same fashion as adipic acid is synthesized from cyclohexane). The diacid is converted to the diamine via the dinitrile.[3]
Uses
TMD is used as a component in certain curing agents for epoxy resins[4].
gollark: [BEE POLL] "Bee Sting" (æææ) <@!353513054912249856>?
gollark: [BEE POLL] Bee Sting (ugh I hate that) <@543131534685765673> (for anti-bee heresy)?
gollark: [BEE POLL] Deploy bees on <@341618941317349376>?
gollark: No.
gollark: Correction: as it's £250 and not the proposed £300, not less, just about the same.
References
- "TRIMETHYLHEXAMETHYLENEDIAMINE". chemicalbook.com. Retrieved 19 April 2015.
- Hardo Siegel, Manfred Eggersdorfer (2005). "Ketones". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a15_077.CS1 maint: uses authors parameter (link)
- U. Rohde-Liebenau (1995). "13.10 PA-TMDT". In Kohan, Melvin (ed.). Nylon Plastics Handbook. Munich: Hanser. p. 570. ISBN 1569901899.
- "Vestamin TMD" (PDF).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.