Triethylphosphine
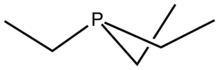
Triethylphosphine is the organophosphorus compound with the formula P(CH2CH3)3, commonly abbreviated as PEt3. It is a colorless liquid with an unpleasant odor characteristic of alkylphosphines. The compound is a common ligand in organometallic chemistry, such as in auranofin.
 | |
| Names | |
|---|---|
| Other names
triethylphosphane | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.245 |
| EC Number |
|
| 2485 | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H15P | |
| Molar mass | 118.160 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.802 g/cm3 |
| Boiling point | 127–128 °C (261–262 °F; 400–401 K) |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H224, H225, H250, H314 |
| P210, P222, P233, P240, P241, P242, P243, P260, P264, P280, P301+330+331, P302+334, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P370+378, P403+235, P405, P422, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure and simple reactions
It is a pyramidal molecule with approximate C3v symmetry.
PEt3 is usually prepared using Grignard reagents:
- 3 CH3CH2MgCl + P(OC6H5)3 → P(CH2CH3)3 + 3 C6H5OMgCl
PEt3 reacts with strong acids to give salts [HPEt3]X.[1] This reaction is reversible. Similarly, it is also easily alkylated to give phosphonium derivatives. PEt3 is easily oxidised to the phosphine oxide with oxygen.
Coordination chemistry
Triethylphosphine is a highly basic ligand that forms coordination complexes with many metals. As a ligand, triethylphosphine's Tolman cone angle is 132°.[2] Being a relatively compact phosphine, several can bind to a single transition metal, as illustrated by the existence of Pt(PEt3)4.[3] As a phosphine ligand, triethylphosphine gained acceptance earlier than did the simpler trimethylphosphine, as illustrated by the preparation of the hydride complex trans-PtHCl(PEt3)2.[4]
Safety
PEt3 is toxic. It converts to a low toxicity phosphine oxide upon treatment with sodium hypochlorite or hydrogen peroxide.
References
- Annette Schier and Hubert Schmidbaur "P-Donor Ligands" in Encyclopedia of Inorganic Chemistry 2006, Wiley-VCH, Weinheim. doi:10.1002/0470862106.ia177
- G. L. Miessler and D. A. Tarr Inorganic Chemistry, 3rd Ed, Pearson/Prentice Hall publisher, ISBN 0-13-035471-6.
- Yoshida, T.; Matsuda, T.; Otsuka, S. (1990). "Tetrakis(Triethylphosphine)Platinum(0)". Inorganic Syntheses. 28: 122. doi:10.1002/9780470132593.ch32.
- Joseph Chatt (1968). "Hydride Complexes". Science. 160: 723–729. doi:10.1126/science.160.3829.723. PMID 17784306.
- Pospiech, S.; Bolte, M.; Lerner, H.-W.; Wagner, M. (2014). "Insertion Reactions into the Boron–Boron Bonds of Barrelene-Type 1,2-Diaminodiboranes(4)". Organometallics. 33: 6967–6974. doi:10.1021/om501087u.CS1 maint: uses authors parameter (link)