Triethylborane
Triethylborane (TEB), also called triethylboron, is an organoborane (a compound with a B-C bond). It is a colorless pyrophoric liquid. Its chemical formula is (C2H5)3B, abbreviated Et3B. It is soluble in organic solvents tetrahydrofuran and hexane.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Triethylborane | |||
| Other names
Triethylborine, triethylboron | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.002.383 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H15B | |||
| Molar mass | 98.00 g/mol | ||
| Appearance | Colorless to pale yellow liquid | ||
| Density | 0.677 g/cm3 | ||
| Melting point | −93 °C (−135 °F; 180 K) | ||
| Boiling point | 95 °C (203 °F; 368 K) | ||
| Not applicable; highly reactive | |||
| Hazards | |||
| Main hazards | Spontaneously flammable in air; causes burns | ||
| Safety data sheet | External SDS | ||
| R-phrases (outdated) | R11 R14/15 R17 R19 R34 R35 R36/37 | ||
| S-phrases (outdated) | S6 S7/8 S16 S33 S36/37/39 S43A S45 S29 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | < −20 °C (−4 °F; 253 K) | ||
| −20 °C (−4 °F; 253 K) | |||
| Related compounds | |||
Related compounds |
| ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Preparation and structure
Triethylborane is prepared by the reaction of trimethyl borate with triethylaluminium:[1]
- Et3Al + (MeO)3B → Et3B + (MeO)3Al
The molecule is monomeric, unlike H3B and Et3Al, which tend to dimerize. It has a planar BC3 core.[1]
Applications
Turbojet engine
Triethylborane was used to ignite the JP-7 fuel in the Pratt & Whitney J58 turbojet/ramjet engines powering the Lockheed SR-71,[2] and its predecessor A-12 OXCART. Triethylborane is suitable for this because of its pyrophoric properties, especially the fact that it burns with a very high temperature. It was chosen as an ignition method for reliability reasons, and in the case of the Blackbird, because the JP-7 fuel has very low volatility and is difficult to ignite. Conventional ignition plugs posed a high risk of malfunction. It was used to start each engine and to ignite the afterburners.[3]
Rocket
Mixed with 10–15% triethylaluminium, it was used before lift-off to ignite the F-1 engines on the Saturn V rocket.[4]
The SpaceX Falcon 9 rocket also uses a triethylaluminium-triethylborane mixture as a first and second stage ignitor.[5]
Organic chemistry
Industrially, triethylborane is used as an initiator in radical reactions, where it is effective even at low temperatures.[1] As an initiator, it can replace some organotin compounds.
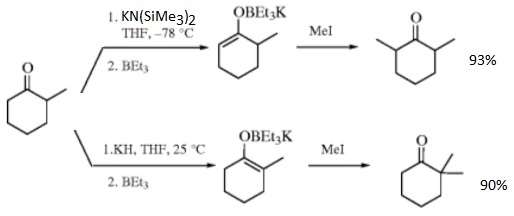
It reacts with metal enolates, yielding enoxytriethylborates that can be alkylated at the α-carbon atom of the ketone more selectively than in its absence. For example, the enolate from treating cyclohexanone with potassium hydride produces 2-allylcyclohexanone in 90% yield when triethylborane is present. Without it, the product mixture contains 43% of the mono-allylated product, 31% di-allylated cyclohexanones, and 28% unreacted starting material.[6] The choice of base and temperature influences whether the more or less stable enolate is produced, allowing control over the position of substituents. Starting from 2-methylcyclohexanone, reacting with potassium hydride and triethylborane in THF at room temperature leads to the more substituted (and more stable) enolate, whilst reaction at −78 °C with potassium hexamethyldisilazide, KN[Si(CH
3)
3]
2 and triethylborane generates the less substituted (and less stable) enolate. After reaction with methyl iodide the former mixture gives 2,2-dimethylcyclohexanone in 90% yield while the latter produces 2,6-dimethylcyclohexanone in 93% yield.[6][7]
It is used in the Barton–McCombie deoxygenation reaction for deoxygenation of alcohols. In combination with lithium tri-tert-butoxyaluminum hydride it cleaves ethers. For example, THF is converted, after hydrolysis, to 1-butanol. It also promotes certain variants of the Reformatskii reaction.[8]
Triethylborane is the precursor to the reducing agents lithium triethylborohydride ("Superhydride") and sodium triethylborohydride.[9]
- MH + Et3B → MBHEt3 (M = Li, Na)
Triethylborane reacts with methanol to form diethyl(methoxy)borane, which is used as the chelating agent in the Narasaka–Prasad reduction for the stereoselective generation of syn-1,3-diols from β-hydroxyketones.[10][11]
Safety
Triethylborane is strongly pyrophoric, with an autoignition temperature of −20 °C (−4 °F),[12] burning with an apple-green flame characteristic for boron compounds. Thus, it is typically handled and stored using air-free techniques.
See also
References
- Brotherton, Robert J.; Weber, C. Joseph; Guibert, Clarence R.; Little, John L. (15 June 2000). "Boron Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a04_309.
- "Lockheed SR-71 Blackbird". March Field Air Museum. Archived from the original on 2000-03-04. Retrieved 2009-05-05.
- "Lockheed SR-71 Blackbird Flight Manual". www.sr-71.org. Retrieved 2011-01-26.
- A. Young (2008). The Saturn V F-1 Engine: Powering Apollo Into History. Springer. p. 86. ISBN 0-387-09629-9.
- Mission Status Center, June 2, 2010, 1905 GMT, SpaceflightNow, accessed 2010-06-02, Quotation: "The flanges will link the rocket with ground storage tanks containing liquid oxygen, kerosene fuel, helium, gaseous nitrogen and the first stage ignitor source called triethylaluminum-triethylborane, better known as TEA-TEB."
- Crich, David, ed. (2008). "Enoxytriethylborates and Enoxydiethylboranes". Reagents for Radical and Radical Ion Chemistry. Handbook of Reagents for Organic Synthesis. 11. John Wiley & Sons. ISBN 9780470065365.
- Negishi, Ei-ichi; Chatterjee, Sugata (1983). "Highly regioselective generation of "thermodynamic" enolates and their direct characterization by NMR". Tetrahedron Letters. 24 (13): 1341–1344. doi:10.1016/S0040-4039(00)81651-2.
- Yamamoto, Yoshinori; Yoshimitsu, Takehiko; Wood, John L.; Schacherer, Laura Nicole (15 March 2007). "Triethylborane". Encyclopedia of Reagents for Organic Synthesis. Wiley. doi:10.1002/047084289X.rt219.pub3.
- Binger, P.; Köster, R. (1974). "Sodium triethylhydroborate, sodium tetraethylborate, and sodium triethyl-1-propynylborate". Inorganic Syntheses. 15: 136–141. doi:10.1002/9780470132463.ch31.
- Chen, Kau-Ming; Gunderson, Karl G.; Hardtmann, Goetz E.; Prasad, Kapa; Repic, Oljan; Shapiro, Michael J. (1987). "A Novel Method for the In situ Generation of Alkoxydialkylboranes and Their Use in the Selective Preparation of 1,3-syn Diols". Chemistry Letters. 16 (10): 1923–1926. doi:10.1246/cl.1987.1923.
- Yang, Jaemoon (2008). "Diastereoselective Syn-Reduction of β-Hydroxy Ketones". Six-Membered Transition States in Organic Synthesis. John Wiley & Sons. pp. 151–155. ISBN 9780470199046.
- Fuels and Chemicals - Autoignition Temperatures