Tricyclohexylphosphine
Tricyclohexylphosphine is the tertiary phosphine with the formula P(C6H11)3. Commonly used as a ligand in organometallic chemistry, it is often abbreviated to PCy3, where Cy stands for cyclohexyl. It is characterized by both high basicity (pKa = 9.7)[1] and a large ligand cone angle (170°).[2][3]
 | |
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Tricyclohexylphosphane | |
| Other names
P(Cy)3 PCy3 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.018.246 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H33P | |
| Molar mass | 280.43 g mol−1 |
| Appearance | white solid |
| Melting point | 82 °C (180 °F; 355 K) |
| organic solvents | |
| Hazards | |
| Main hazards | toxic |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Important complexes containing P(Cy)3 ligands include the 2005 Nobel Prize-winning Grubbs' catalyst and the homogeneous hydrogenation catalyst Crabtree's catalyst.
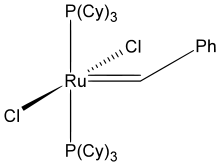 Grubbs' catalyst (first generation)
Grubbs' catalyst (first generation)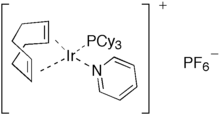 Crabtree's catalyst
Crabtree's catalyst
References
- Streuli, C. A. (1960). "Determination of Basicity of Substituted Phosphines by Nonaqueous Titrimetry". Anal. Chem. 32: 985–987. doi:10.1021/ac60164a027.
- Bush, R. C.; Angelici, R. J. (1988). "Phosphine basicities as determined by enthalpies of protonation". Inorg. Chem. 27 (4): 681–686. doi:10.1021/ic00277a022.
- Immirzi, A.; Musco, A. (1977). "A Method to Measure the Size of Phosphorus Ligands in Coordination Complexes". Inorg. Chim. Acta. 25: L41–42. doi:10.1016/S0020-1693(00)95635-4.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.