trans-1,2-Diaminocyclohexane
trans-1,2-Diaminocyclohexane is an organic compound with the formula C6H10(NH2)2. This diamine is a building block for C2-symmetric ligandss that are useful in asymmetric catalysis.[1]
 | |
| Names | |
|---|---|
| IUPAC name
(±)-trans-1,2-Cyclohexanediamine | |
| Other names
1,2-Diaminocyclohexane; chxn | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.127.756 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C6H14N2 | |
| Molar mass | 114.192 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.951 g/cm3 |
| Melting point | 14 to 15 °C (57 to 59 °F; 287 to 288 K) |
| Boiling point | 79 to 81 °C (174 to 178 °F; 352 to 354 K) 15 mm Hg |
| Hazards | |
| Flash point | 69 °C; 156 °F; 342 K |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
A mixture of cis- and trans-1,2-diaminocyclohexane is produced by the hydrogenation of o-phenylenediamine. It is also side product in hydrogenation of adiponitrile. The racemic trans isomer (1:1 mixture of (1R,2R)-1,2-diaminocyclohexane and (1S,2S)-1,2-diaminocyclohexane) can be separated into the two enantiomers using enantiomerically pure tartaric acid as the resolving agent.[2]
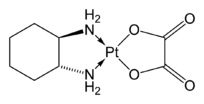
Derived ligands
Representative ligands prepared from (1R,2R)- or (1S,2S)-1,2-diaminocyclohexane are diaminocyclohexanetetraacetic acid (CyDTAH4), Trost ligand, and the salen analogue used in the Jacobsen epoxidation.
References
- Kouklovsky, Cyrille; Langlois, Yves (2003). "(1S,2S)-1,2-Diaminocyclohexane". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00145. ISBN 0471936235.
- Jay F. Larrow and Eric N. Jacobsen (2004). "(R,R)-N,N'-Bis(3,5-Di-tert-Butylsalicylidene)-1,2-Cyclohexanediamino Manganese(III) Chloride, A Highly Enantioselective Epoxidation Catalyst". Organic Syntheses.; Collective Volume, 10, p. 96