Timeline of tuberous sclerosis
The history of tuberous sclerosis (TSC) research spans less than 200 years. TSC is a rare, multi-system genetic disease that can cause benign tumours to grow on the brain or other vital organs such as the kidneys, heart, eyes, lungs, and skin. A combination of symptoms may include seizures, developmental delay, behavioural problems and skin abnormalities, as well as lung and kidney disease. TSC is caused by mutations on either of two genes, TSC1 and TSC2, which encode for the proteins hamartin and tuberin respectively. These proteins act as tumour growth suppressors and regulate cell proliferation and differentiation.[1] Originally regarded as a rare pathological curiosity, it is now an important focus of research into tumour formation and suppression.

The history of TSC research is commonly divided into four periods.[2] In the late 19th century, notable physicians working in European teaching hospitals first described the cortical and dermatological manifestations; these early researchers have been awarded with eponyms such as "Bourneville's disease"[3] and "Pringle's adenoma sebaceum".[4] At the start of the 20th century, these symptoms were recognised as belonging to a single medical condition. Further organ involvement was discovered, along with a realisation that the condition was highly variable in its severity. The late 20th century saw great improvements in cranial imaging techniques and the discovery of the two genes. Finally, the start of the 21st century saw the beginning of a molecular understanding of the illness, along with possible non-surgical therapeutic treatments.
19th century
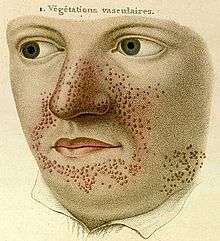
- 1835
- French dermatologist Pierre François Olive Rayer published an atlas of skin diseases. It contains 22 large coloured plates with 400 figures presented in a systematic order. On page 20, fig. 1 is a drawing that is regarded as the earliest description of tuberous sclerosis.[5] Entitled "végétations vasculaires", Rayer noted these were "small vascular, of papulous appearance, widespread growths distributed on the nose and around the mouth".[6] No mention was made of any medical condition associated with the skin disorder.
- 1850
- English dermatologists Thomas Addison and William Gull described, in Guy's Hospital Reports, the case of a four-year-old girl with a "peculiar eruption extending across the nose and slightly affecting both cheeks", which they called "vitiligoidea tuberosa".[7]
- 1862
- German physician Friedrich Daniel von Recklinghausen, who was working as an assistant to Rudolf Virchow in the Institute for Pathological Anatomy in Berlin,[8] presented a case to the city's Obstetrical Society.[9] The heart of an infant who "died after taking a few breaths" had several tumours. He called these tumours "myomata", one of which was the "size of a pigeon's egg".[7] He also noted the brain had "a great number of scleroses".[5] These were almost certainly the cardiac rhabdomyomas and cortical tubers of tuberous sclerosis. He failed to recognise a distinct disease, regarding it as a pathological-anatomical curiosity.[10] Von Recklinghausen's name would instead become associated with neurofibromatosis after a classic paper in 1881.[8]
- 1864
- German pathologist Rudolf Virchow published a three-volume work on tumours that described a child with cerebral tuberous sclerosis and rhabdomyoma of the heart. His description contained the first hint that this may be an inherited disease: the child's sister had died of a cerebral tumour.[11]
- 1880
- French neurologist Désiré-Magloire Bourneville had a chance encounter with the disease that would bear his name. He was working as an unofficial assistant to Jean Martin Charcot at La Salpêtrière.[10] While substituting for his teacher, Louis J.F. Delasiauve,[12] he attended to Marie, a 15-year-old girl with psychomotor retardation, epilepsy and a "confluent vascular-papulous eruption of the nose, the cheeks and forehead". She had a history of seizures since infancy and was taken to the children's hospital aged three and declared a hopeless case. She had learning difficulties and could neither walk nor talk. While under Bourneville's care, Marie had an ever-increasing number of seizures, which came in clusters. She was treated with quinquina, bromide of camphor, amyl nitrite, and the application of leeches behind the ears. On 7 May 1879 Marie died in her hospital bed. The post-mortem examination disclosed hard, dense tubers in the cerebral convolutions, which Bourneville named Sclérose tubéreuse des circonvolutions cérébrales. He concluded they were the source (focus) of her seizures. In addition, whitish hard masses, one "the size of a walnut", were found in both kidneys.[13]
- 1881
- German physician Hartdegen described the case of a two-day-old baby who died in status epilepticus. Post-mortem examination revealed small tumours in the lateral ventricles of the brain and areas of cortical sclerosis, which he called "glioma gangliocellulare cerebri congenitum".[14][15]
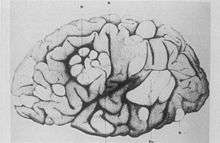
- 1881
- Bourneville and Édouard Brissaud examined a four-year-old boy at La Bicêtre. As before, this patient had cortical tubers, epilepsy and learning difficulties. In addition he had a heart murmur and, on post-mortem examination, had tiny hard tumours in the ventricle walls in the brain (subependymal nodules) and small tumours in the kidneys (angiomyolipomas).[16]
- 1885
- French physicians Félix Balzer and Pierre Eugène Ménétrier reported a case of "adénomes sébacés de la face et du cuir" (adenoma of the sebaceous glands of the face and scalp).[17] The term has since proved to be incorrect as they are neither adenoma nor derived from sebaceous glands. The papular rash is now known as facial angiofibroma.[18]
- 1885
- French dermatologists François Henri Hallopeau and Émile Leredde published a case of adenoma sebaceum that was of a hard and fibrous nature. They first described the shagreen plaques and later would note an association between the facial rash and epilepsy.[7][19]
- 1890
- Scottish dermatologist John James Pringle, working in London, described a 25-year-old woman with subnormal intelligence, rough lesions on the arms and legs, and a papular facial rash. Pringle brought attention to five previous reports, two of which were unpublished.[20] Pringle's adenoma sebaceum would become a common eponym for the facial rash.
Early 20th century

.jpg)
- 1901
- Italian physician GB Pellizzi studied the pathology of the cerebral lesions. He noted their dysplastic nature, the cortical heterotopia and defective myelination. Pellizzi classified the tubers into type 1 (smooth surface) and type 2 (with central depressions).[21][22]
- 1903
- German physician Richard Kothe described periungual fibromas, which were later rediscovered by the Dutch physician Johannes Koenen in 1932 (known as Koenen's tumours).[23]
- 1906
- Australian neurologist Alfred Walter Campbell, working in England, considered the lesions in the brain, skin, heart and kidney to be caused by one disease. He also first described the pathology in the eye. His review of 20 reported cases led him to suggest a diagnostic triad of symptoms that is more commonly attributed to Vogt.[24]
- 1908
- German paediatric neurologist Heinrich Vogt established the diagnostic criteria for TSC, firmly associating the facial rash with the neurological consequences of the cortical tubers.[25][26] Vogt's triad of epilepsy, idiocy, and adenoma sebaceum held for 60 years until research by Manuel Gómez discovered that fewer than a third of patients with TSC had all three symptoms.[5]
- 1910
- J. Kirpicznick was first to recognise that TSC was a genetic condition. He described cases of identical and fraternal twins and also one family with three successive generations affected.[27]
- 1911
- Edward Sherlock, barrister-at-law and lecturer in biology, reported nine cases in his book on the "feeble-minded". He coined the term epiloia, a portmanteau of epilepsy and anoia (mindless).[28] The word is no longer widely used as a synonym for TSC. The geneticist Robert James Gorlin suggested in 1981 that it could be a useful acronym for epilepsy, low intelligence, and adenoma sebaceum.[29]
- 1913
- H. Berg is credited with first stating that TSC was a hereditary disorder, noting its transmission through two or three generations.[30]
- 1914
- P. Schuster described a patient with adenoma sebaceum and epilepsy but of normal intelligence.[7] This reduced phenotypic expression is called a forme fruste.[31]
- 1918
- French physician René Lutembacher published the first report of cystic lung disease in a patient with TSC. The 36-year-old woman died from bilateral pneumothoraces. Lutembacher believed the cysts and nodules to be metastases from a renal fibrosarcoma. This complication, which only affects women, is now known as lymphangioleiomyomatosis (LAM).[32][33]
- 1920
- Dutch ophthalmologist Jan van der Hoeve described the retinal hamartomas (phakoma). He grouped both TSC and neurofibromatosis together as "phakomatoses" (later called neurocutaneous syndromes).[34]
Mid-20th century
- 1932
- MacDonald Critchley and Charles J.C. Earl studied 29 patients with TSC who were in mental institutions. They described behaviour—unusual hand movements, bizarre attitudes and repetitive movements (stereotypies)—that today would be recognised as autistic. However it would be 11 years before Leo Kanner suggested the term "autism". They also noticed the associated white spots on the skin (hypomelanic macules).[36]
- 1934
- N.J. Berkwitz and L.G. Rigler showed it was possible to diagnose tuberous sclerosis using pneumoencephalography to highlight non-calcified subependymal nodules. These resembled "the wax drippings of a burning candle" on the lateral ventricles.[37]
- 1942
- Sylvan E. Moolten proposed "the tuberous sclerosis complex", which is now the preferred name. This recognises the multi-organ nature of the disease. Moolten introduced three words to describe its pathology: "the basic lesion is hamartial, becoming in turn tumor-like (hamartoma) or truly neoplastic (hamartoblastoma)."[38]
- 1954
- Norwegian pathologist Reidar Eker bred a line of Wistar rats predisposed to renal adenomas. The Eker rat became an important model of dominantly inherited cancer.[39]
- 1966
- Phanor Perot and Bryce Weir pioneered surgical intervention for epilepsy in TSC. Of the seven patients who underwent cortical tuber resection, two became seizure-free. Prior to this, only four patients had ever been surgically treated for epilepsy in TSC.[40]
- 1967
- J.C. Lagos and Manuel Rodríguez Gómez reviewed 71 TSC cases and found that 38% of patients have normal intelligence.[14][41]
- 1971
- American geneticist Alfred Knudson developed his "two hit" hypothesis to explain the formation of retinoblastoma in both children and adults. The children had a congenital germline mutation which was combined with an early lifetime somatic mutation to cause a tumour. This model applies to many conditions involving tumour suppressor genes such as TSC.[42] In the 1980s, Knudson's studies on the Eker rat strengthened this hypothesis.[43]
- 1975
- Giuseppe Pampiglione and E. Pugh, in a letter to The Lancet, noted that up to 69% of patients presented with infantile spasms.[44]
- 1975
- Riemann first used ultrasound to examine TSC-affected kidneys in the case of a 35-year-old woman with chronic renal failure.[45]
Late 20th century
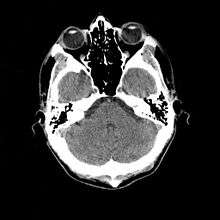
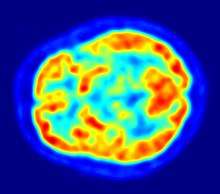
- 1976
- Cranial computed tomography (CT, invented 1972) proved to be an excellent tool for diagnosing cerebral neoplasms in children, including those found in tuberous sclerosis.[46]
- 1977
- Ann Mercy Hunt MBE and others found the Tuberous Sclerosis Association in the UK to provide self help and to fund research.[47]
- 1979
- Manuel Gómez published a monograph: "Tuberous Sclerosis" that remained the standard textbook for three editions over two decades. The book described the full clinical spectrum of TSC for the first time and established a new set of diagnostic criteria to replace the Vogt triad.[14][48]
- 1982
- Kenneth Arndt successfully treated facial angiofibroma with an argon laser.[49]
- 1983
- Positron emission tomography (PET, invented 1981) was compared to electroencephalography (EEG) and CT. It was found to be capable of locating epileptogenic cortical tubers that would otherwise have been missed.[50]
- 1984
- The cluster of infantile spasms in TSC was discovered to be preceded by a focal EEG discharge.[51]
- 1985
- Magnetic resonance imaging (MRI, invented 1980) was first used in TSC to identify affected regions in the brain of a girl with tuberous sclerosis.[52]
- 1987
- MR was judged superior to CT imaging for both sensitivity and specificity. In a study of fifteen patients, it identified subependymal nodules projecting into the lateral ventricles in twelve patients, distortion of the normal cortical architecture in ten patients (corresponding to cortical tubers), dilated ventricles in five patients, and distinguished a known astrocytoma from benign subependymal nodules in one patient.[53]
- 1987
- MR imaging was found to be capable of predicting the clinical severity of the disease (epilepsy and developmental delay). A study of 25 patients found a correlation with the number of cortical tubers identified. In contrast, CT was not a useful predictor, but was superior at identifying calcified lesions.[54]
- 1987
- Linkage analysis on 19 families with TSC located a probable gene on chromosome 9.[55]
- 1988
- Cortical tubers found on MR imaging corresponded exactly to the location of persistent EEG foci, in a study of six children with TSC. In particular, frontal cortical tubers were associated with more intractable seizures.[56]
- 1990
- Vigabatrin was found to be a highly effective antiepileptic treatment for infantile spasms, particularly in children with TSC.[57] Following the discovery in 1997 of severe persistent visual field constriction as a possible side-effect, vigabatrin monotherapy is now largely restricted to this patient group.[58]
- 1992
- Linkage analysis located a second gene to chromosome 16p13.3, close to the polycystic kidney disease type 1 (PKD1) gene.[59]
- 1993
- The European Chromosome 16 Tuberous Sclerosis Consortium announced the cloning of TSC2; its product is called tuberin.[60]
- 1994
- The Eker rat was discovered to be an animal model for tuberous sclerosis; it has a mutation in the rat-equivalent of the TSC2 gene.[61]
- 1995
- MRI with fluid attenuated inversion recovery (FLAIR) sequences was reported to be significantly better than standard T2-weighted images at highlighting small tubers, especially subcortical ones.[62][63]
- 1997
- The TSC1 Consortium announced the cloning of TSC1; its product is called hamartin.[64]
- 1997
- The PKD1 gene, which leads to autosomal dominant polycystic kidney disease (ADPKD), and the TSC2 gene were discovered to be adjacent on chromosome 16p13.3. A team based at the Institute of Medical Genetics in Wales studied 27 unrelated patients with TSC and renal cystic disease. They concluded that serious renal disease in those with TSC is usually due to contiguous gene deletions of TSC2 and PKD1. They also noted that the disease was different (earlier and more severe) than ADPKD and that patients with TSC1 did not suffer significant cystic disease.[65]
- 1997
- Patrick Bolton and Paul Griffiths examined 18 patients with TSC, half of whom had some form of autism. They found a strong association between tubers in the temporal lobes and the patients with autism.[66]
- 1998
- The Tuberous Sclerosis Consensus Conference issued revised diagnostic criteria.[67]
- 1998
- An Italian team used magnetoencephalography (MEG) to study three patients with TSC and partial epilepsy. Combined with MRI, they were able to study the association between tuberous areas of the brain, neuronal malfunctioning and epileptogenic areas.[68] Later studies would confirm that MEG is superior to EEG in identifying the eliptogenic tuber, which may be a candidate for surgical resection.[69]
21st century
- 2001
- A multi-centre cohort of 224 patients were examined for mutations and disease severity. Those with TSC1 were less severely affected than those with TSC2. They had fewer seizures and less mental impairment. Some symptoms of TSC were rare or absent in those with TSC1. A conclusion is that "both germline and somatic mutations appear to be less common in TSC1 than in TSC2".[70]
- 2002
- Several research groups investigated how the TSC1 and TSC2 gene products (tuberin and hamartin) work together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signalling. This important pathway regulates cell proliferation and tumour suppression.[71]
- 2002
- Treatment with rapamycin (sirolimus) was found to shrink tumours in the Eker rat (TSC2)[72] and mouse (TSC1)[73] models of tuberous sclerosis.
- 2006
- Small trials showed promising results in the use of rapamycin to shrink angiomyolipoma[74] and astrocytomas.[75] Several larger multicentre clinical trials began: lymphangioleiomyomatosis (LAM)[76] and kidney angiomyolipoma (AML)[77] were treated with rapamycin; giant cell astrocytomas were treated with the rapamycin derivative everolimus.[78]
2012
A consensus conference was held and revised guidelines for the diagnosis and management of tuberous sclerosis were published.[79][80]
Notes
- "Tuberous Sclerosis Fact Sheet". NINDS. 11 April 2006. Archived from the original on 21 January 2007. Retrieved 9 January 2007.
- Rott (2005), page 2 - Introduction.
- Enersen OD. "Désiré-Magloire Bourneville". Who Named It?. Retrieved 30 April 2007.
- Enersen OD. "John James Pringle". Who Named It?. Retrieved 30 April 2007.
- Curatolo (2003), chapter: "Historical Background".
- Rayer PF (1835). Traité des maladies de la peau / atlas (in French). Paris: J.B. Baillière. p. 20. Retrieved 9 December 2006.
- Jay V (2004). "Tuberous sclerosis". Pediatric and Developmental Pathology. 2 (2): 197–8. doi:10.1007/s100249900110. PMID 9949228.
- Enersen OD. "Friedrich Daniel von Recklinghausen". Who Named It?. Retrieved 10 December 2006.
- von Recklinghausen F (1862). "Ein Herz von einem Neugeborene welches mehrere theils nach aussen, theils nach den Höhlen prominirende Tumoren (Myomen) trug". Monatschr Geburtsheilkd (in German). 20: 1–2.(As cited in Curatolo (2003))
- Jansen FE, van Nieuwenhuizen O, van Huffelen AC (May 2004). "Tuberous sclerosis complex and its founders". Journal of Neurology, Neurosurgery, and Psychiatry. 75 (5): 770. doi:10.1136/jnnp.2003.027524. PMC 1763558. PMID 15090576.
- Virchow R (July 1863). Die Krankhaften Geschwülste. Vol II. Berlin: August Hirschwald. p. 148.(As cited in Acierno (1994))
- Wilkins, Robert H (ed); Brody, Irwin A (ed) (1997). "XXXI Tuberous Sclerosis". Neurological Classics. American Association of Neurological Surgeons. pp. 149–52. ISBN 978-1-879284-49-4.CS1 maint: multiple names: authors list (link) CS1 maint: extra text: authors list (link) (contains an abridged translation of Bourneville's 1880 paper)
- Bourneville D (1880). "Sclérose tubéreuse des circonvolutions cérébrales: Idiotie et épilepsie hemiplégique". Archives de Neurologie (in French). 1: 81–9l. Retrieved 22 August 2009.
- Sancak Ö (2005). Tuberous Sclerosis Complex: Mutations, Functions and Phenotypes. Stichting Tubereuze Sclerose Nederland. pp. 11–2. ISBN 978-90-902019-3-1.
- Hartdegen A (February 1881). "Ein Fall von multipler Verhärtung des Grosshirns nebst histologisch eigenartigen harten Geschwülsten der Seitenventrikel ("Glioma gangliocellulare") bei einem Neugeborenen". European Archives of Psychiatry and Clinical Neuroscience. 11 (1): 117–31. doi:10.1007/BF02054825.
- Bourneville D, Brissaud É (1881). "Encéphalite ou sclérose tubéreuse des circonvolutions cérébrales". Archives de Neurologie. 1: 390–412.(As cited in Curatolo (2003))
- Balzer F, Ménétrier P (1885). "Étude sur un cas d'adénomes sébacés de la face et du cuir". Archives de Physiologie Normale et Pathologique. Third Series. 6: 564–76.(As cited in Curatolo (2003))
- Sanchez NP, Wick MR, Perry HO (December 1981). "Adenoma sebaceum of Pringle: a clinicopathologic review, with a discussion of related pathologic entities". Journal of Cutaneous Pathology. 8 (6): 395–403. doi:10.1111/j.1600-0560.1981.tb01028.x. PMID 6278000.
- Hallopeau F, Leredde É (1885). "Sur un cas d'adenomes sébacés à forme sclereuse". Ann Dermatol Syph. 6: 473–9.(As cited in Curatolo (2003))
- Pringle JJ (1890). "A case of congenital adenoma sebaceum". British Journal of Dermatology. 2: 1–14. Retrieved 22 August 2009.
- Pellizzi GB (1901). "Contributo allo studio dell'idiozia: rivisita sperimentale di freniatria e medicina legale delle alienazioni mentali". Riv Sper Freniat. 27: 265–9.(As cited in Curatolo (2003))
- Braffman BH, Bilaniuk LT, Naidich TP, Altman NR, Post MJ, Quencer RM, Zimmerman RA, Brody BA (April 1992). "MR imaging of tuberous sclerosis: pathogenesis of this phakomatosis, use of gadopentetate dimeglumine, and literature review". Radiology. 183 (1): 227–38. doi:10.1148/radiology.183.1.1549677. PMID 1549677.
- Kothe R (1903). "Zur Lehre der Talgdrüsengeschwülste". Archiv für Dermatologie und Syphilis (in German). 68 (3): 273–8. doi:10.1007/BF01829939.(As cited in Rott (2005))
- Campbell AW (1906). "Cerebral sclerosis". Brain. 28 (3–4): 382–96. doi:10.1093/brain/28.3-4.367.
- Enersen OD. "Heinrich Vogt". Who Named It?. Retrieved 11 December 2006.
- Vogt H (1908). "Zur Diagnostik der tuberösen Sklerose". Zeitschrift für die Erforschung und Behandlung des Jugendlichen Schwachsinns Auf Wissenschaftlicher Grundlage, Jena. 2: 1–16.(As cited in Curatolo (2003) and fully cited by Who Named It?)
- Kirpicznik J (1910). "Ein Fall von Tuberoser Sklerose und gleichzeitigen multiplen Nierengeschwùlsten". Virchows Archiv für pathologische Anatomie und Physiologie und für klinische Medizin (in German). 202 (3): 358–376. doi:10.1007/BF01993975.(As cited in Curatolo (2003))
- Sherlock EB (1911). The Feeble-minded, A Guide to Study and Practice. Macmillan & Co.(As cited in Jay (2004))
- Online Mendelian Inheritance in Man (OMIM): Tuberous Sclerosis - 191100
- Berg H (1913). "Vererbung der tuberösen Sklerose durch zwei bzw. drei Generationen". Z Ges Neurol Psychiatr (in German). 19: 528–39. doi:10.1007/BF02909909.(As cited in Curatolo (2003))
- Schuster P (1914). "Beiträge zur Klinik der tuberösen Sklerose des Gehirns". Dtsch Z Nervenheilkd (in German). 50: 96–133.(As cited in Curatolo (2003))
- Lutembacher R (1918). "Dysembryomes métatypique des reins. Carcinose submiliaire aigue du poumon avec emphysème généralisé et double pneumothorax". Annals of Medicine (in French). 5: 435–50.(As cited in Curatolo (2003))
- Abbott GF, Rosado-de-Christenson ML, Frazier AA, Franks TJ, Pugatch RD, Galvin JR (2005). "From the archives of the AFIP: lymphangioleiomyomatosis: radiologic-pathologic correlation". Radiographics. 25 (3): 803–28. doi:10.1148/rg.253055006. PMID 15888627.
- Van der Hoeve J (1920). "Eye symptoms in tuberous sclerosis of the brain". Trans Ophthalmol Soc UK. 40: 329–34.(As cited in Curatolo (2003))
- Marcus H (1924). Svenska Làk Sallsk Forth.(As cited by Dickerson WW (1945). "Characteristic roentgenographic changes associated with tuberous sclerosis". Archives of Neurology and Psychiatry. 53 (3): 199–204. doi:10.1001/archneurpsyc.1945.02300030036005., as cited in Curatolo (2003) and Gómez (1995))
- Critchley M, Earl CJ (1932). "Tuberose sclerosis and allied conditions". Brain. 55 (3): 311–46. doi:10.1093/brain/55.3.311.
- Berkwitz NJ, Rigler LG (1934). "Tuberous sclerosis diagnosed with cerebral pneumography". Archives of Neurology and Psychiatry. 35: 833–8.(As cited in Gómez (1995))
- Moolten SE (1942). "Hamartial nature of tuberous sclerosis complex and its bearings on the tumor problem: report of a case with tumor anomaly of the kidney and adenoma sebaceum". Arch Intern Med. 69 (4): 589–623. doi:10.1001/archinte.1942.00200160040005.
- Eker R (1954). "Familial renal adenomas in Wistar rats; a preliminary report". Acta Pathologica et Microbiologica Scandinavica. 34 (6): 554–62. doi:10.1111/j.1699-0463.1954.tb00301.x. PMID 13206757.(As cited in Yeung (1994))
- Perot P, Weir B, Rasmussen T (November 1966). "Tuberous sclerosis. Surgical therapy for seizures". Archives of Neurology. 15 (5): 498–506. doi:10.1001/archneur.1966.00470170052005. PMID 5955139.(As cited in Bebin EM, Kelly PJ, Gomez MR (1993). "Surgical treatment for epilepsy in cerebral tuberous sclerosis". Epilepsia. 34 (4): 651–7. doi:10.1111/j.1528-1157.1993.tb00442.x. PMID 8330575.)
- Lagos JC, Gomez MR (January 1967). "Tuberous sclerosis: reappraisal of a clinical entity". Mayo Clinic Proceedings. 42 (1): 26–49. PMID 5297238.(As cited in Curatolo (2003))
- Knudson AG (April 1971). "Mutation and cancer: statistical study of retinoblastoma". Proceedings of the National Academy of Sciences of the United States of America. 68 (4): 820–3. Bibcode:1971PNAS...68..820K. doi:10.1073/pnas.68.4.820. PMC 389051. PMID 5279523.(As cited in Rott (2005))
- Yeung RS (December 2004). "Lessons from the Eker rat model: from cage to bedside". Current Molecular Medicine. 4 (8): 799–806. doi:10.2174/1566524043359791. PMID 15579026.
- Pampiglione G, Pugh E (November 1975). "Letter: Infantile spasms and subsequent appearance of tuberous sclerosis syndrome". Lancet. 2 (7943): 1046. doi:10.1016/S0140-6736(75)90343-8. PMID 53537.
- Riemann JF, Mörl M, Rott HD (June 1975). "[Chronic renal failure in bourneville-pringle's disease (author's transl)]". Medizinische Klinik (in German). 70 (26): 1128–32. PMID 1223616.(As cited in Rott (2005))
- Berger PE, Kirks DR, Gilday DL, Fitz CR, Harwood-Nash DC (July 1976). "Computed tomography in infants and children: intracranial neoplasms". AJR. American Journal of Roentgenology. 127 (1): 129–37. doi:10.2214/ajr.127.1.129. PMID 180824.
- McFarlane, Isobel (2014-07-30). "Ann Hunt obituary". The Guardian. ISSN 0261-3077. Retrieved 2019-08-14.
- Gómez MR (1979). Tuberous Sclerosis (1st ed.). New York: Raven Press. ISBN 978-0-89004-313-4.(As cited in Özgür (2005))
- Arndt KA (July 1982). "Adenoma sebaceum: successful treatment with the argon laser". Plastic and Reconstructive Surgery. 70 (1): 91–3. doi:10.1097/00006534-198207000-00021. PMID 7089113.(As cited in Rott (2005))
- Szelies B, Herholz K, Heiss WD, Rackl A, Pawlik G, Wagner R, Ilsen HW, Wienhard K (December 1983). "Hypometabolic cortical lesions in tuberous sclerosis with epilepsy: demonstration by positron emission tomography". Journal of Computer Assisted Tomography. 7 (6): 946–53. doi:10.1097/00004728-198312000-00002. PMID 6415136.(As cited in Rott (2005))
- Dulac O, Lemaitre A, Plouin P (1984). "The Bourneville syndrome: clinical and EEG features of epilepsy in the first year of life". Boll Lega Ital Epil. 45/46: 39–42.(As cited in Curatolo (2003))
- Kandt RS, Gebarski SS, Goetting MG (August 1985). "Tuberous sclerosis with cardiogenic cerebral embolism: magnetic resonance imaging". Neurology. 35 (8): 1223–5. doi:10.1212/wnl.35.8.1223. PMID 4022361.(As cited in Rott (2005))
- McMurdo SK, Moore SG, Brant-Zawadzki M, Berg BO, Koch T, Newton TH, Edwards MS (April 1987). "MR imaging of intracranial tuberous sclerosis". AJR. American Journal of Roentgenology. 148 (4): 791–6. doi:10.2214/ajr.148.4.791. PMID 3493666.
- Roach ES, Williams DP, Laster DW (March 1987). "Magnetic resonance imaging in tuberous sclerosis". Archives of Neurology. 44 (3): 301–3. doi:10.1001/archneur.1987.00520150047020. PMID 3827681.(As cited in Curatolo (2003))
- Fryer AE, Chalmers A, Connor JM, Fraser I, Povey S, Yates AD, Yates JR, Osborne JP (March 1987). "Evidence that the gene for tuberous sclerosis is on chromosome 9". Lancet. 1 (8534): 659–61. doi:10.1016/S0140-6736(87)90416-8. PMID 2882085.
- Curatolo P, Cusmai R (September 1988). "[Magnetic resonance imaging in Bourneville's disease: relation to the EEG]". Neurophysiologie Clinique = Clinical Neurophysiology (in French). 18 (5): 459–67. doi:10.1016/s0987-7053(88)80056-x. PMID 3185465.(As cited in Curatolo (2003))
- Chiron C, Dulac O, Luna D, Palacios L, Mondragon S, Beaumont D, Mumford JP (February 1990). "Vigabatrin in infantile spasms". Lancet. 335 (8685): 363–4. doi:10.1016/0140-6736(90)90660-W. PMID 1967808.
- Vigabatrin Paediatric Advisory Group (May 2000). "Guideline for prescribing vigabatrin in children has been revised. Vigabatrin Paediatric Advisory Group". BMJ. 320 (7246): 1404–5. doi:10.1136/bmj.320.7246.1404. PMC 1118061. PMID 10858057.
- Kandt RS, Haines JL, Smith M, Northrup H, Gardner RJ, Short MP, Dumars K, Roach ES, Steingold S, Wall S (September 1992). "Linkage of an important gene locus for tuberous sclerosis to a chromosome 16 marker for polycystic kidney disease". Nature Genetics. 2 (1): 37–41. doi:10.1038/ng0992-37. PMID 1303246.
- European Chromosome 16 Tuberous Sclerosis Consortium (December 1993). "Identification and characterization of the tuberous sclerosis gene on chromosome 16". Cell. 75 (7): 1305–15. doi:10.1016/0092-8674(93)90618-Z. PMID 8269512.(As cited in Rott (2005))
- Yeung RS, Xiao GH, Jin F, Lee WC, Testa JR, Knudson AG (November 1994). "Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene". Proceedings of the National Academy of Sciences of the United States of America. 91 (24): 11413–6. Bibcode:1994PNAS...9111413Y. doi:10.1073/pnas.91.24.11413. PMC 45241. PMID 7972075.
- Maeda M, Tartaro A, Matsuda T, Ishii Y (1995). "Cortical and subcortical tubers in tuberous sclerosis and FLAIR sequence". Journal of Computer Assisted Tomography. 19 (4): 660–1. doi:10.1097/00004728-199507000-00033. PMID 7622707.
- Takanashi J, Sugita K, Fujii K, Niimi H (October 1995). "MR evaluation of tuberous sclerosis: increased sensitivity with fluid-attenuated inversion recovery and relation to severity of seizures and mental retardation". AJNR. American Journal of Neuroradiology. 16 (9): 1923–8. PMID 8693996.
- van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, et al. (August 1997). "Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34". Science. 277 (5327): 805–8. doi:10.1126/science.277.5327.805. PMID 9242607.
- Sampson JR, Maheshwar MM, Aspinwall R, Thompson P, Cheadle JP, Ravine D, Roy S, Haan E, Bernstein J, Harris PC (October 1997). "Renal cystic disease in tuberous sclerosis: role of the polycystic kidney disease 1 gene". American Journal of Human Genetics. 61 (4): 843–51. doi:10.1086/514888. PMC 1716004. PMID 9382094.
- Bolton PF, Griffiths PD (February 1997). "Association of tuberous sclerosis of temporal lobes with autism and atypical autism". Lancet. 349 (9049): 392–5. doi:10.1016/S0140-6736(97)80012-8. PMID 9033466.
- Roach ES, Gomez MR, Northrup H (December 1998). "Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria". Journal of Child Neurology. 13 (12): 624–8. doi:10.1177/088307389801301206. PMID 9881533.
- Peresson M, Lopez L, Narici L, Curatolo P (October 1998). "Magnetic source imaging and reactivity to rhythmical stimulation in tuberous sclerosis". Brain & Development. 20 (7): 512–8. doi:10.1016/S0387-7604(98)00034-5. PMID 9840671.
- Jansen FE, Huiskamp G, van Huffelen AC, Bourez-Swart M, Boere E, Gebbink T, Vincken KL, van Nieuwenhuizen O (January 2006). "Identification of the epileptogenic tuber in patients with tuberous sclerosis: a comparison of high-resolution EEG and MEG". Epilepsia. 47 (1): 108–14. doi:10.1111/j.1528-1167.2006.00373.x. PMID 16417538. Archived from the original on 2012-12-17.
- Dabora SL, Jozwiak S, Franz DN, Roberts PS, Nieto A, Chung J, Choy YS, Reeve MP, Thiele E, Egelhoff JC, Kasprzyk-Obara J, Domanska-Pakiela D, Kwiatkowski DJ (January 2001). "Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs". American Journal of Human Genetics. 68 (1): 64–80. doi:10.1086/316951. PMC 1234935. PMID 11112665.
- Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J (October 2002). "Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling". Proceedings of the National Academy of Sciences of the United States of America. 99 (21): 13571–6. Bibcode:2002PNAS...9913571T. doi:10.1073/pnas.202476899. PMC 129715. PMID 12271141.
- Kenerson HL, Aicher LD, True LD, Yeung RS (October 2002). "Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors". Cancer Research. 62 (20): 5645–50. PMID 12384518.
- Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H (March 2002). "A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells". Human Molecular Genetics. 11 (5): 525–34. doi:10.1093/hmg/11.5.525. PMID 11875047.
- Wienecke R, Fackler I, Linsenmaier U, Mayer K, Licht T, Kretzler M (September 2006). "Antitumoral activity of rapamycin in renal angiomyolipoma associated with tuberous sclerosis complex". American Journal of Kidney Diseases. 48 (3): e27-9. doi:10.1053/j.ajkd.2006.05.018. PMID 16931204.
- Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G, Dinopoulos A, Thomas G, Crone KR (March 2006). "Rapamycin causes regression of astrocytomas in tuberous sclerosis complex". Annals of Neurology. 59 (3): 490–8. doi:10.1002/ana.20784. PMID 16453317. Archived from the original on 2012-12-17.
- "Multicenter International Lymphangioleiomyomatosis Efficacy of Sirolimus Trial (The MILES Trial)". ClinicalTrials.gov (NIH). 6 January 2007. Retrieved 10 January 2007.
- "Sirolimus in Treating Patients With Angiomyolipoma of the Kidney". ClinicalTrials.gov (NIH). 21 November 2006. Retrieved 10 January 2007.
- "Everolimus (RAD001) Therapy of Giant Cell Astrocytoma in Patients With Tuberous Sclerosis Complex". ClinicalTrials.gov (NIH). 13 December 2006. Retrieved 10 January 2007.
- Krueger DA, Northrup H (October 2013). "Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference". Pediatric Neurology. 49 (4): 255–65. doi:10.1016/j.pediatrneurol.2013.08.002. PMC 4058297. PMID 24053983.
- Northrup H, Krueger DA (October 2013). "Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference". Pediatric Neurology. 49 (4): 243–54. doi:10.1016/j.pediatrneurol.2013.08.001. PMC 4080684. PMID 24053982.
References
- Acierno LJ (1994). The History of Cardiology. Taylor & Francis. p. 427. ISBN 978-1-85070-339-6.
- Curatolo P, ed. (2003). Tuberous Sclerosis Complex : From Basic Science to Clinical Phenotypes. MacKeith Press. ISBN 978-1-898683-39-1.
- Gómez MR (1995). "History of the tuberous sclerosis complex". Brain & Development. 17 Suppl (suppl): 55–7. doi:10.1016/0387-7604(94)00130-8. PMID 8882573.
- Rott HD, Mayer K, Walther B, Wienecke R (2005). "Zur Geschichte der Tuberösen Sklerose (The History of Tuberous Sclerosis)" (PDF) (in German). Tuberöse Sklerose Deutschland e.V. Archived from the original (PDF) on 15 March 2007. Retrieved 8 January 2007.