Thiosulfinate
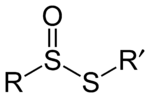
In organosulfur chemistry, thiosulfinate is a functional group consisting of the linkage R-S(O)-S-R (R are organic substituents). Thiolsulfinates are also named as alkanethiosulfinic (or arenethiosulfinic) acid esters. They are the first member of a family of compounds containing an oxidized disulfide bond. Other members of this family include thiosulfonates (R-SO2-S-R), α-disulfoxides (R-S(O)-S(O)-R), sulfinyl sulfones (R-S(O)-SO2-R), and α-disulfones (R-SO2-SO2-R), all of which are known. The thiosulfinate group can occur in cyclic as well as acyclic structures.[1][2][3]
Occurrence
A variety of acyclic and cyclic thiosulfinates are found in plants, or formed when the plants are cut or crushed. A well-known thiosulfinate is allicin, CH2=CHCH2S(O)SCH2CH=CH2, one of the active ingredients formed when garlic is crushed. Allicin was discovered in 1944 by Chester J. Cavallito and coworkers. Thiosulfinates containing various combinations of the methyl, n-propyl, 1-propenyl, 2-propenyl, n-butyl, 1-butenyl and 2-butenyl groups are formed upon crushing different Allium as well as Brassica species.[4][5] Crushing the roots of Petiveria alliacea affords the thiosulfinates S-(2-hydroxyethyl) 2-hydroxyethane)thiosulfinate, S-(2-hydroxylethyl) phenylmethanethiosulfinate, S-benzyl 2-hydroxyethane)thiosulfinate and S-benzyl phenylmethanethiosulfinate (petivericin; PhCH2S(O)SCH2Ph).[6] Zeylanoxides are cyclic thiosulfinates containing the 1,2-dithiolane-1-oxide ring, isolated from the tropical weed Sphenoclea zeylanica. These heterocyclic thiosulfinates are chiral at carbon as well as at sulfur.[7] Asparagusic acid S-oxide[8] and brugierol[9] are other natural 1,2-dithiolane-1-oxides occurring in Asparagus officinalis and Brugiera conjugata, respectively.
Properties
Allicin, S-benzyl phenylmethanethiosulfinate, and related thiosulfinates show radical-trapping antioxidant activity associated with easy formation of sulfenic acids[10] The acyclic thiosulfinates from Allium and Brassica species possess antimicrobial, antiparasitic, antitumor and cysteine protease inhibitory activity while the natural 1,2-dithiolane-1-oxides are growth inhibitors. The thiosulfinates from Petiveria also exhibit antimicrobial activity.[11] Thiosulfinates feature a S(IV) center linked to a S(II) center, the former being stereogenic. Conversion of simple disulfides to thiosulfinates results in a considerable weakening of the S–S bond from about 70 to 34.5 kcal mol−1 (16.7 to 8.25 kJ mol−1) for the S-S bond in PhS(O)SPh,[12] with the consequence that most thiosulfinates are both unstable and quite reactive. For this reason the mixtures of thiosulfinates from Allium plants can best be separated by HPLC at room temperature rather than by gas chromatography (GC), although GC has been used with some low molecular weight thiosulfinates. Thiosulfinates can be distinguished from sulfoxides by infrared spectroscopy since they have a characteristic S=O band at about 1078 cm−1 compared to 1030–1060 cm−1 in sulfoxides.[13]
Formation and reactions
The first synthesis of thiosulfinates was reported in 1947 by Cavallito and coworkers by oxidation of the corresponding disulfides.[14] One example of a moderately stable thiosulfinate is the tert-butyl derivative, (CH3)3CS(O)SC(CH3)3. This thiosulfinate can be obtained in optical purity by catalytic asymmetric oxidation of di-tert-butyl disulfide with hydrogen peroxide.[15] Upon heating, (CH3)3CS(O)SC(CH3)3 decomposes into tert-butanethiosulfoxylic acid (CH3)3CSSOH) as shown by trapping studies.[16] In a similar manner racemic methyl methanethiosulfinate (CH3S(O)SCH3) can be obtained by peracetic acid oxidation of dimethyl disulfide.[17] Methyl methanethiosulfinate decomposes thermally giving methanesulfenic acid (CH3SOH), the simplest sulfenic acid, as well as thioformaldehyde (CH2=S). Methyl methanethiosulfinate can also disproportionate to a 1:1 mixture of dimethyl disulfide and methyl methanethiosulfonate (CH3SO2SCH3) and rearrange via a Pummerer rearrangement to CH3S(O)CH2SSCH3.[18][19] An unusual three-membered ring thiosulfinate (a dithiirane 1-oxide) has been prepared through rearrangement of a 1,3-dithietane.[20] A related compound, 3-(9-triptycyl)dithiirane-1-oxide, was prepared by the reaction of (9-triptycyl)diazomethane and S8O. The X-ray structure of the dithiirane-1-oxide reveals a significantly lengthened sulfur-sulfur bond (211.9(3)pm).[21] Thiosulfinates have also been invoked as intermediates in the oxidation of thiols to sulfonic acids.
References
- Kice JL (1980). "Mechanisms and reactivity in reactions of organic oxyacids of sulfur and their anhydrides". Advances in Physical Organic Chemistry. 17: 65–181. doi:10.1016/S0065-3160(08)60128-8.
- Takata, T; Endo, T (1990). "Thiosulphinic acids and esters". The Chemistry of Sulphinic Acids, Esters and Their Derivatives, S. Patai, Ed. (John Wiley, NY): 527–575. doi:10.1002/9780470772270.ch18.
- Braverman, S; Cherkinsky, M.; Levinger, S. (2007). "Alkanethiosulfinic Acid Esters". Sci. Synth. 39: 229–235.
- Kubec, R; Cody, RB; Dane, AJ; Musah, RA; Schraml, J; Vattekkatte, A; Block, E (2010). "Applications of DART Mass Spectrometry in Allium Chemistry. (Z)-Butanethial S-Oxide and 1-Butenyl Thiosulfinates and their S-(E)-1-Butenylcysteine S-Oxide Precursor from Allium siculum". J. Agric. Food Chem. 58 (2): 1121–1128. doi:10.1021/jf903733e. PMID 20047275.
- Block, E; Dane, AJ; Thomas, S; Cody, RB (2010). "Applications of Direct Analysis in Real Time–Mass Spectrometry (DART-MS) in Allium Chemistry. 2-Propenesulfenic and 2-Propenesulfinic Acids, Diallyl Trisulfane S-Oxide and Other Reactive Sulfur Compounds from Crushed Garlic and Other Alliums". J. Agric. Food Chem. 58 (8): 4617–4625. doi:10.1021/jf1000106. PMID 20225897.
- Kubec, R; Kim, S; Musah, RA (2002). "S-Substituted cysteine derivatives and thiosulfinate formation in Petiveria alliacea--Part II" (PDF). Phytochemistry. 61: 675–680. doi:10.1016/S0031-9422(02)00328-X.
- Hirai, N; Sakashita, S; Sano, T; Inoue, T; Ohigashi, H; Premasthira, C; Asakawa, Y; Harada, J; Fujii, Y (2000). "Allelochemicals of the tropical weed Sphenoclea zeylanica". Phytochemistry. 55: 131–140. doi:10.1016/S0031-9422(00)00264-8.
- Yanagawa, H; Kato, T; Kitahara, Y (1973). "Asparagusic acid-S-oxides, new plant growth regulators in etiolated young asparagus shoots". Tetrahedron Letters. 14: 1073–1075. doi:10.1016/S0040-4039(01)95907-6.
- Kato, A; Numata M (1972). "Brugierol and isobrugierol, trans- and cis-1,2-dithiolane-1-oxide, from Brugiera conjugata". Tetrahedron Letters. 13: 203–206. doi:10.1016/S0040-4039(01)84280-5.
- Lynett, PT; Butts, K; Vaidya, V; Garretta, GE; Pratt, DA (2011). "The mechanism of radical-trapping antioxidant activity of plant-derived thiosulfinates". Org. Biomol. Chem. 9: 3320–3330. doi:10.1039/c1ob05192j.
- Kim, S; Kubec, R; Musah, RA (2006). "Antibacterial and antifungal activity of sulfur-containing compounds from Petiveria alliacea" (PDF). Journal of Ethnopharmacology. 104: 188–192. doi:10.1016/j.jep.2005.08.072. PMID 16229980.
- Koch, P; Ciuffarin, E; Fava, A (1970). "Thermal disproportionation of aryl arenethiolsulfinates. Kinetics and mechanism". J. Am. Chem. Soc. 92: 5971–5977. doi:10.1021/ja00723a026.
- Block E (2010). Garlic and Other Alliums: The Lore and the Science. Royal Society of Chemistry. ISBN 0-85404-190-7.
- Small, LD; Bailey, JH; Cavallito, CJ (1947). "Alkyl thiolsulfinates". J. Am. Chem. Soc. 69: 1710–1713. doi:10.1021/ja01199a040.
- Weix, DJ; Ellman, JA (2005). "(RS)-(+)-2-Methyl-2-Propanesulfinamide [tert-Butanesulfinamide]". Organic Syntheses. 82: 157. doi:10.1002/0471264229.os082.24.
- Block, E (1972). "The Chemistry of Alkyl Thiosulfinate Esters. III. tert-Butanethiosulfoxylic Acid". J. Am. Chem. Soc. 94: 644–645. doi:10.1021/ja00757a060.
- Moore, TL; O'Connor, DE (1966). "The Reaction of Methanesulfenyl Chloride with Alkoxides and Alcohols. Preparation of Aliphatic Sulfenate and Sulfinate Esters". J. Org. Chem. 31: 3587–3592. doi:10.1021/jo01349a027.
- Block, E; O'Connor, J (1974). "The Chemistry of Alkyl Thiosulfinate Esters. VI. Preparation and Spectral Studies". J. Am. Chem. Soc. 96: 3921–3929. doi:10.1021/ja00819a033.
- Block, E; O'Connor, J (1974). "The Chemistry of Alkyl Thiosulfinate Esters. VII. Mechanistic Studies and Synthetic Applications". J. Am. Chem. Soc. 96: 3929–3944. doi:10.1021/ja00819a034.
- Ishii, A; Akazawa, T; Ding, MX; Honjo, T; Nakayama, J; Hoshino, M; Shiro, M (1993). "First isolable dithiiranes: 3-(1,1,3,3-tetramethyl-4-oxo-4-phenylbutyl)-3-phenyldithiirane 1-oxides". J. Am. Chem. Soc. 115: 4914–4915. doi:10.1021/ja00064a072.
- Ishii, A; Kawai, T; Noji, M; Nakayama, J (2005). "Synthesis and reactions of a monosubstituted dithiirane 1-oxide, 3-(9-triptycyl)dithiirane 1-oxide". Tetrahedron. 61: 6693–6699. doi:10.1016/j.tet.2005.05.017.