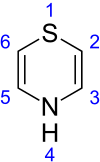
Thiazine
Thiazine /ˈθaɪəziːn/ is an organic compound containing a ring of four carbon, one nitrogen and one sulfur atom. There are three isomers of thiazine, 1,2-thiazine, 1,3-thiazine, and 1,4-thiazine, which differ by the arrangement of the nitrogen and sulfur atoms in the ring.
 | |
| Names | |
|---|---|
| IUPAC name
4H-1,4-Thiazine | |
| Other names
Parathiazine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| |
| |
| Properties | |
| C4H5NS | |
| Molar mass | 99.15 g·mol−1 |
| Density | 0.8465 g/cm3 |
| Boiling point | 76.5 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
1,4-thiazine can be prepared from the corresponding dione using aluminium powder at high temperature.[1]

Synthesis of 1,4-thiazine.
Derivatives
Derivatives of thiazine, often referred to as thiazines, are used for dyes, tranquilizers and insecticides.
gollark: They're microscopic bees you can't see.
gollark: They don't use mind control *beams* though, they use mind control *bees*, which aren't blocked by metal.
gollark: Yes you do. They just use mind control to make you *think* you don't.
gollark: They're only visible if you wear Google Glass, which is why the government banned that.
gollark: To fake gravity, obviously, and disappear people they don't like.
See also
- Methylene blue, contains a related ring with nitrogen and a positively charged sulfur atom
- Phenothiazine, a thiazine fused with two benzene rings
- Thiomorpholine, a saturated analog of thiazine
References
- Barkenbus, Charles; Landis, Phillip S. (February 1948). "The Preparation of 1,4-Thiazine". Journal of the American Chemical Society. 70 (2): 684–685. doi:10.1021/ja01182a075. ISSN 0002-7863.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
