Thiadiazoles
In chemistry thiadiazoles are a sub-family of azole compounds. Structurally they are five-membered heterocyclic compounds containing two nitrogen and a sulfur atoms, and two double bonds, to give an aromatic ring; with the name thiadiazole originating from the Hantzsch–Widman nomenclature. Four possible structures exist depending on the relative positions of the heteroatoms; these forms do not interconvert and hence are structural isomers and not tautomers. The compounds themselves are rarely synthesized and possess no particular application, however compounds bearing them as a structural motif are fairly common in pharmacology.[1][2][3]
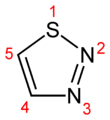 1,2,3-Thiadiazole
1,2,3-Thiadiazole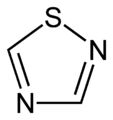 1,2,4-thiadiazole
1,2,4-thiadiazole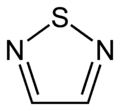 1,2,5-thiadiazole
1,2,5-thiadiazole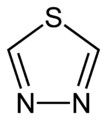 1,3,4-thiadiazole
1,3,4-thiadiazole
References
- Hu, Yang; Li, Cui-Yun; Wang, Xiao-Ming; Yang, Yong-Hua; Zhu, Hai-Liang (2014). "1,3,4-Thiadiazole: Synthesis, Reactions, and Applications in Medicinal, Agricultural, and Materials Chemistry". Chemical Reviews. 114 (10): 5572–5610. doi:10.1021/cr400131u. ISSN 0009-2665. PMID 24716666.
- Jain, Abhishek Kumar; Sharma, Simant; Vaidya, Ankur; Ravichandran, Veerasamy; Agrawal, Ram Kishore (2013). "1,3,4-Thiadiazole and its Derivatives: A Review on Recent Progress in Biological Activities". Chemical Biology & Drug Design. 81 (5): 557–576. doi:10.1111/cbdd.12125. ISSN 1747-0277.

- Wim Dehaen; Vasiliy A. Bakulev; Edward C. Taylor; Jonathan A. Ellman (27 April 2004). The Chemistry of Heterocyclic Compounds, The Chemistry of 1,2,3-Thiadiazoles. John Wiley & Sons. pp. 5–. ISBN 978-0-471-65691-3.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.