Tetrakis(dimethylamino)ethylene
Tetrakis(dimethylamino)ethylene (TDAE) is an organic compound with the formula C2(N(CH3)2)4. It is a colorless liquid. It is classified as an enamine.[1]
4.png) | |
| Names | |
|---|---|
| IUPAC name
1-N,1-N,1-N',1-N',2-N,2-N,2-N',2-N'-octamethylethene-1,1,2,2-tetramine | |
| Other names
Octamethyl-ethenetetramine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.012.398 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H24N4 | |
| Molar mass | 200.330 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.861 g/cm3 (25 °C) |
| Melting point | −4 °C (25 °F; 269 K) |
| Boiling point | 59 °C (0.9 mm Hg) |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
GHS hazard statements |
H226, H314 |
| P210, P233, P240, P241, P242, P243, P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P370+378, P403+235, P405, P501 | |
| Flash point | 53 °C (127 °F; 326 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Reactions
TDAE reacts with oxygen in a chemiluminscent reaction to give tetramethylurea[2][3]
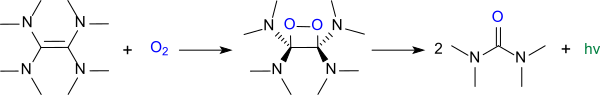
Oxidation of TDAE (chemiluminescence).
TDAE is an electron donor. It forms a charge transfer salt with buckminsterfullerene:[4]
- C2(N(CH3)2)4 + C60 → [C2(N(CH3)2)4+][C60−]
gollark: Aaagh why.
gollark: https://dragcave.net/teleport/b9bfbe6f122801a07bd920a327365664
gollark: Oops, wrong URL.
gollark: Seen on trade hub - cheap 2G prize.https://dragcave.net/teleport/1410efa5b0825595c9cf8a11219c51fe
gollark: Perhaps, perhaps.
References
- David M. Lemal (1968). "Tetraaminoethylenes". In Saul Patai (ed.). The Amino Group. pp. 701–748. doi:10.1002/9780470771082. ISBN 9780470771082.
- H.E. Winberg; J. R. Downing; D. D. Coffman (1965). "The Chemiluminescence of Tetrakis(dimethylamino)ethylene". J. Am. Chem. Soc. 87: 2054–2055. doi:10.1021/ja01087a039.
- "Chemilumineszenz von TDAE" (in German). illumina-chemie.de. 2014-08-08. Retrieved 2016-08-22.
- Allemand, P.-M.; Khemani, K. C.; Koch, A.; Wudl, F.; Holczer, K.; Donovan, S.; GrÜner, G.; Thompson, J. D. (1991). "Organic Molecular Soft Ferromagnetism in a Fullerene". Science. 253: 301. doi:10.1126/science.253.5017.301.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.