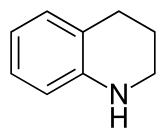
Tetrahydroquinoline
Tetrahydroquinoline is an organic compound that is the semi-hydrogenated derivative of quinoline. It is a colorless oil.
 | |
| Names | |
|---|---|
| IUPAC name
1,2,3,4-Tetrahydroquinoline | |
| Other names
Hydroquinoline | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.010.216 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C9H11N | |
| Molar mass | 133.194 g·mol−1 |
| Appearance | Colorless oily liquid |
| Density | 1.0599 g/cm3 |
| Melting point | 20 °C (68 °F; 293 K) |
| Boiling point | 251 °C (484 °F; 524 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Use
Substituted derivatives of tetrahydroquinoline are common in medicinal chemistry.[1] Oxamniquine, dynemycin, viratmycin, and nicainoprol are bioactive tetrahydroquinolines.[2] Typically tetrahydroquinoline derivatives are prepared by hydrogenation of the corresponding quinoline using heterogeneous catalysts.
Synthesis
Tetrahydroquinolines are produced by hydrogenation of quinolines. Because the hydrogenation is reversible, tetrahydroquinoline has been often examined as a hydrogen-donor solvent in coal liquifaction.
Using homogeneous catalysts, asymmetric hydrogenation has been demonstrated.[3] It can also be prepared from 1-indanone (benzocyclopentanone).[4]
References
- Sridharan, Vellaisamy; Suryavanshi, Padmakar A.; Menéndez, J. Carlos (2011). "Advances in the Chemistry of Tetrahydroquinolines". Chemical Reviews. 111 (11): 7157–7259. doi:10.1021/cr100307m. PMID 21830756.
- Katritzky, Alan R.; Rachwal, Stanislaw; Rachwal, Bogumila (1996). "Recent Progress in the Synthesis of 1,2,3,4-Tetrahydroquinolines". Tetrahedron. 52 (48): 15031–15070. doi:10.1016/S0040-4020(96)00911-8.
-
Chen, Fei; Ding, Zi-Yuan; He, Yan-Mei; Fan, Qing-Hua (2015). "Synthesis of Optically Active 1,2,3,4-Tetrahydroquinolines via Asymmetric Hydrogenation Using Iridium-Diamine Catalyst". 92: 213–226. doi:10.15227/orgsyn.092.0213. Cite journal requires
|journal=(help) - Imaizumi, Taku; Okano, Kentaro; Tokuyama, Hidetoshi (2016). "DIBALH-Mediated Reductive Ring-Expansion Reaction of Cyclic Ketoxime". 93: 1–13. doi:10.1002/0471264229.os093.01. Cite journal requires
|journal=(help)