Tetrachlorozincate
Tetrachlorozincate is an anion with the formula [ZnCl4]2−. It is a counterion that is often used in conjunction with strong electrophiles. Being dianionic, tetrachlorozincate is not classified as a weakly coordinating anion. On the other hand, being dianionic, tetrachlorozincate facilitates the crystallization of many salts. The anion is tetrahedral. Zincates are anionic zinc complexes.
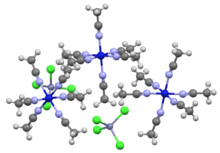
Portion of the crystal structure of the tetrachlorozincate (ZnCl42−) salt of [Ni(MeCN)6]2+.[1]
Related to the preparation of Lucas' reagent, tetrachlorozincates are often generated by combining hydrochloric acid and zinc chloride.
A related anion is [Zn2Cl6]2−, in which again Zn(II) adopts a tetrahedral geometry.
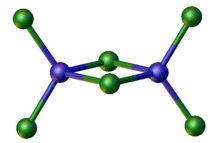
The structure of [Zn2Cl6]2−.[2]
References
- I. Sotofte, R. G. Hazell, S. E. Rasmussen (1976). "Hexaacetonitrilenickel(II) tetrachlorozincate. A crystal structure with serious overlap in the Patterson function". Acta Crystallographica Section B. 32: 1692. doi:10.1107/S0567740876006249.CS1 maint: uses authors parameter (link)
- F. A. Cotton, S.A.Duraj, W.J.Roth (1985). "Two Compounds Containing the Tris(μ-chloro)hexakis(tetrahydrofuran)divanadium(II) Cation. Preparation, Structures, and Spectroscopic Characterization". Inorg. Chem. 24: 913. doi:10.1021/ic00200a023.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.