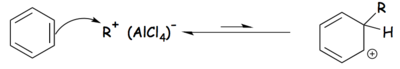Tetrachloroaluminate
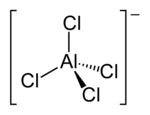
Tetrachloroaluminate [AlCl4]− is an anion formed from aluminium and chlorine. The anion has a tetrahedral shape, similar to carbon tetrachloride where carbon is replaced with aluminium. Some tetrachloroaluminates are soluble in organic solvents, creating an ionic non-aqueous solution, making them suitable as component of electrolytes for batteries. E.g. lithium tetrachloroaluminate is used in some lithium batteries.
 | |
| Names | |
|---|---|
| IUPAC name
Tetrachloroaluminate(1–) | |
| Systematic IUPAC name
Tetrachloroaluminate(1-) | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| 2297 | |
PubChem CID |
|
| |
| |
| Properties | |
| AlCl4− | |
| Molar mass | 168.78 g·mol−1 |
| Structure | |
| Td | |
| Tetrahedral | |
| Hybridisation | sp3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Formation
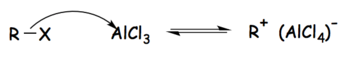
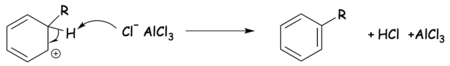
Tetrachloroaluminate ions are formed as intermediaries in the Friedel–Crafts alkylation reaction, in which Aluminium chloride is used as a catalyst. The Friedel-Crafts alkylation reaction can be broken into three steps, as follows:[1]
Step 1: The alkyl halide reacts with the strong Lewis acid to form an activated electrophile composed of the tetrachloroaluminate ion and the alkyl group.
Step 2: The aromatic ring (benzene in this case) reacts with the activated electrophile forming an alkyl-benzenonium carbocation.
Step 3: The alkyl-benzenonium carbocation reacts with a tetrachloroaluminate anion, regenerating the aromatic ring and the Lewis acid and forming hydrochloric acid (HCl).
References
- "Electrophilic substitution - the alkylation of benzene". chemguide. Retrieved 28 July 2020.