Tetrabenzylzirconium
Tetrabenzylzirconium is an organozirconium compound with the formula Zr(CH2C6H5)4. The molecule features diamagnetic Zr(IV) bonded to four benzyl ligands. It is a yellow air- and photo-sensitive solid, which is soluble in hydrocarbon solvents. The compound is a precursor to catalysts for the polymerization of olefins.[1] [2]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C28H28Zr | |
| Molar mass | 455.756 g·mol−1 |
| Appearance | yellow solid |
| Melting point | 133–134 °C (271–273 °F; 406–407 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure, synthesis, reactions
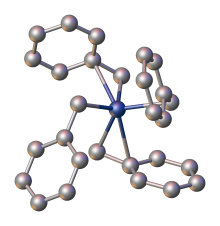
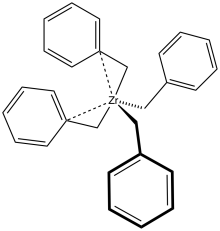
Structure of tetrabenzylzirconium as determined by X-ray crystallography, with H atoms omitted for clarity.[3]
X-ray crystallography demonstrates that the benzyl ligands are highly flexible: one polymorph features four η2-ligands, whereas another has two η1- and two η2-benzyl ligands.[3]
The compound is prepared by combining benzylmagnesium chloride and zirconium tetrachloride in diethyl ether. It readily undergoes protonolysis, e.g. with hydrogen chloride:
- Zr(CH2C6H5)4 + HCl → Zr(CH2C6H5)3Cl + CH3C6H5
gollark: Consumer monitors are just bad. They can't even display 11MeV X-rays.
gollark: You can install a V"P"S (manually) on:- a Raspberry Pi 3B- the osmarks.net primary server, an old HP tower- osmarksVPS™- my Kindle, assuming it is on the wireless network
gollark: We have a range of locations available.
gollark: You could always use osmarksV"P"S™ plans.
gollark: I see.
References
- Tshuva, Edit Y.; Goldberg, Israel; Kol, Moshe (2000). "Isospecific Living Polymerization of 1-Hexene by a Readily Available Nonmetallocene C2-Symmetrical Zirconium Catalyst". Journal of the American Chemical Society. 122 (43): 10706–10707. doi:10.1021/ja001219g.
- Zucchini, U.; Albizzati, E.; Giannini, U. (1971). "Synthesis and Properties of Some Titanium and Zirconium Benzyl Derivatives". Journal of Organometallic Chemistry. 26: 357–372. doi:10.1016/S0022-328X(00)82618-2.
- Rong, Yi; Al-Harbi, Ahmed; Parkin, Gerard (2012). "Highly Variable Zr–CH2–Ph Bond Angles in Tetrabenzylzirconium: Analysis of Benzyl Ligand Coordination Modes". Organometallics. 31 (23): 8208–8217. doi:10.1021/om300820b.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.