Tantalate
Tantalate is an tantalum-containing anion or a salt of such an anion. A commercially important example is heptafluorotantalate (TaF72−) and its potassium salt (K2TaF7).
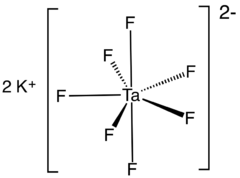 Potassium heptafluorotantalate.
Potassium heptafluorotantalate.
Many oxides of tantalum are called tantalates. They are viewed as derivatives of "tantalic acid", hypothetic compounds with the formulas Ta2O5·nH2O[1] or HTaO3[2]). Examples of such tantalates are lithium tantalate (LiTaO3), lutetium tantalate (LuTaO4) and lead scandium tantalate (PST or Pb(ScxTa1-x)O3. Polyoxometallates containing tantalum provide examples of discrete tantalum oxides that exist in solution.
References
- Szanics, Judit; Kakihana, Masato (1999). "A Novel Tantalic Acid-Based Polymerizable Complex Route to LiTaO3 Using Neither Alkoxides nor Chlorides of Tantalum". Chemistry of Materials. 11 (10): 2760. doi:10.1021/cm990160d.
- Inoue, Y (1996). "Synthetic inorganic ion exchange materials XLI: Ion exchange properties of cubic tantalic acid (HTaO3)". Materials Research Bulletin. 31 (6): 691. doi:10.1016/0025-5408(96)00050-5.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.